Abstract -
Introduction -
Materials and Methods -
Results -
Discussion -
References
Abstract
Eight classes of chemosensory neurons in C. elegans fill with fluorescein when living animals are placed in a dye solution. Fluorescein enters the neurons through their exposed sensory cilia. Mutations in 14 genes prevent dye uptake and disrupt chemosensory behaviors. Each of these genes affects the ultrastructure of the chemosensory cilia or their accessory cells. In each case, the cilia are shorter or less exposed than normal, suggesting that dye contact is the principal factor under selection. Ten genes affect many or all of the sensory cilia in the head. The
daf-19 (m86) mutation eliminates all cilia, leaving only occasional centrioles in the dendrites. The cilia in
che-13 (e1805), osm-1 (p808), osm-5 (p813), and osm-6 (p811) mutants have normal transition zones and severely shortened axonemes. Doublet-microtubules, attached to the membrane by Y links, assemble ectopically proximal to the cilia in these mutants. The amphid cilia in
che-11 (e1810) are irregular in diameter and contain dark ground material in the middle of the axonemes. Certain mechanocilia are also affected. The amphid cilia in
che-10 (e1809) apparently degenerate, leaving dendrites with bulb-shaped endings filled with dark ground material. The mechanocilia lack striated rootlets. Cilia defects have also been found in che-2, che-3, and daf-10 mutants. The
osm-5 (p802) mutation specifically eliminates the distal segment of the amphid cilia. Mutations in three genes affect sensillar support cells. The
che-12 (e1812) mutation eliminates matrix material normally secreted by the amphid sheath cell. The
che-14 (e1960) mutation disrupts the joining of the amphid sheath and socket cells to form the receptor channel. A similar defect has been observed in daf-6 mutants. Four additional genes affect specific classes of ciliated sensory neurons. The
mec-1 and mec-8 (e398) mutations disrupt the fasciculation of the amphid cilia. The
cat-6 (e1861) mutation disrupts the tubular bodies of the CEP mechanocilia. A cryophilic thermotaxis mutant,
ttx-1 (p767), lacks fingers on the AFD dendrite, suggesting this neuron is thermosensory. © 1986 Academic Press, Inc.
Introduction
Cilia and flagella are ubiquitous eukaryotic organelles that have been adapted for two seemingly unrelated functions, sensory transduction and cell motility. In the unicellular eukaryotes, Chlamydomonas and
Paramecium, for example, they are used for swimming. Similarly, flagella propel the sperm of many animals and lower plants. Arrays of motile cilia line various epithelia, including the respiratory tracts, the oviducts, and the ventricles of the brain, where they propel fluid or particles along the surface.
Sensory cilia are found in the rod and cone cells of the eye,
the hair cells of the ear, and the olfactory receptor neurons. In nematodes,
cilia are found only in the nervous system where they are sensory receptors
specialized for diverse modalities (Ward
et al, 1975; Ware et al,
1975). Of the 118 classes of neurons in Centralists elegans hermaphrodites,
24 classes have cilia (White et al,
1986).
The common plan of both motile and sensory cilia is a membrane-bound cylinder of nine doublet microtubules that extend from a centriole. Many cilia have additional structures that adapt them to specific tasks. As they are biochemically complex structures and, in many cases, present in limited numbers, genetic studies have been helpful in understanding the assembly and function of cilia (Afzelius, 1981). In
Chlamydomonas and Paramecium, genes coding for ciliary proteins have been identified by selecting for mutants with abnormal swimming (Luck, 1984; Kung et al, 1975). In humans, genetic disorders of ciliary motility produce a syndrome of male infertility and respiratory distress (Afzelius, 1976).
In C. elegans, several collections of mutants have been obtained by selecting for altered sensory behavior (Dusenbery et al, 1975; Hedgecock and Russell, 1975; Lewis and Hodgkin, 1977; Culotti and Russell, 1978; Chalfie and Sulston, 1981; Riddle et al, 1981; Hodgkin, 1983; and Trent et al, 1983). While some of these mutations affect the sensory organs themselves (Lewis and Hodgkin, 1977; Albert et al, 1981; Chalfie and Sulston, 1981; R. Ware, D. Dusenbery, D. Clark, M. Szalay, and R. Russell, personal communication), others presumably disrupt behavior at steps downstream of transduction.
Recently we found that certain sensory neurons in C. elegans
accumulate fluorescein when living animals are placed in a solution of this
dye (Hedgecock et al, 1985). In this paper, we show that these neurons are chemosensory
and that dye uptake occurs through their exposed cilia. We have used this dye-filling
technique to identify a subset of behavioral mutants with primary defects in
sensory cilia or their support cells. These mutations both prevent dye uptake
and disrupt sensory behaviors.
Materials and Methods
Brenner (1974) describes culturing and genetic manipulation of Caenorhabditis elegans. Strains were kindly provided by Martin Chalfie, David Dusenbery, Jonathan Hodgkin, Donald Riddle, Richard Russell, and the Caenorhabditis Genetics Center at the University of Missouri, Columbia.
Chemosensory neurons were stained with fluorescein isothiocyanate
and examined by fluorescence microscopy as in Hedgecock et al (1985). New mutants
with abnormal staining were induced with ethylmethanesulphonate (Brenner, 1974).
The cat-6 (e1861) mutation was separated from the strain CB246.
Animals were fixed for electron microscopy using glutaraldehyde and then osmium as in Sulston et al. (1983). Usually, two or three individuals of each mutant strain were embedded and sectioned together. About 200 contiguous sections, 50 nm thick, were collected from the tips of the heads. One animal, selected for good fixation quality and orientation, was photographed approximately every third section at 5000X magnification to reconstruct the head sensilla. The other animals were examined directly in the microscope.
Results
Description of the Amphid and Phasmid Sensilla
The amphids, a pair of lateral sensilla in the head, are the principal chemosensory
organs of nematodes (Fig. 1). In C. elegans, each amphid comprises the
ciliated dendrites of 12 sensory neurons plus two support cells called sheath
and socket cells (Ward et al, 1975; Ware et al, 1975; White et al, 1986). The
sheath and socket cells form a cylindrical channel to the outside (Fig. 2).
Of the 12 amphid neurons, 8 (ASE,
ADF, ASG,
ASH, ASI,
ASJ, ASK,
and ADL) are evidently chemosensory
in that their cilia extend into the channel of the socket cell and are thereby
exposed to the external medium. The cilia of three additional neurons (AWA,
AWB, and AWC),
called wing cells, also share the main lumen of the sheath cell. The wing cilia
separate from the others, and invaginate individually into the sheath cell,
proximal to where the fascicle of channel cilia enters the socket cell. Finally,
the dendrite of a neuron (AFD),
called the finger cell, remains separate from the other dendrites in the sheath
cell. It has only a rudimentary cilium but, proximal to the cilium, the dendritic
membrane expands into about fifty villi, called fingers, that invaginate the
sheath cell (Fig. 2). These fingers are about 0.15 micrometer in diameter and
2 micrometer long. No internal microfilaments or microtubules have been seen
in them but they tend to be oriented anteriorly or posteriorly in the sheath
cell.
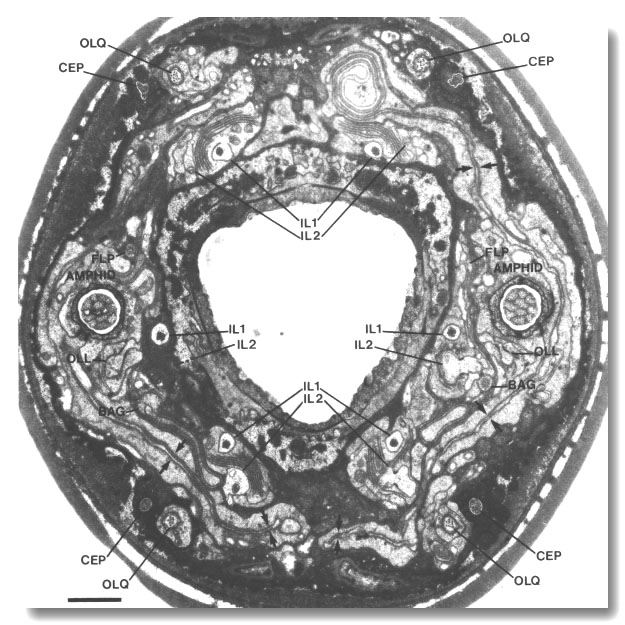
Figure 1. Anterior sensilla in wild-type hermaphrodite. Section
4.0 micrometer from tip of head. The fascicles of amphid channel cilia
(AMPHID), positioned laterally, have just entered the socket channels. The wings
of the AWC cilia (arrows)
are spread vertically in the amphid sheath cell. Six pairs of inner labial dendrites
(IL1 and IL2)
invaginate the inner labial sheath cells. A large striated rootlet is visible
in each IL1 dendrite. Dorsally and ventrally, the four CEP
and four OLQ cilia are sectioned
through their middle segments. The squares of microtubules in the OLQ
cilia are oriented with corners circumferential and radial. The two OLL
dendrites, positioned laterally, are sectioned through their junctions with
sheath cells. The cilia of the BAG
and FLP neurons are also
visible. The left FLP cilium
and the right BAG cilium
are sectioned through their transition zones. Scale bar is 1.0 micrometer.
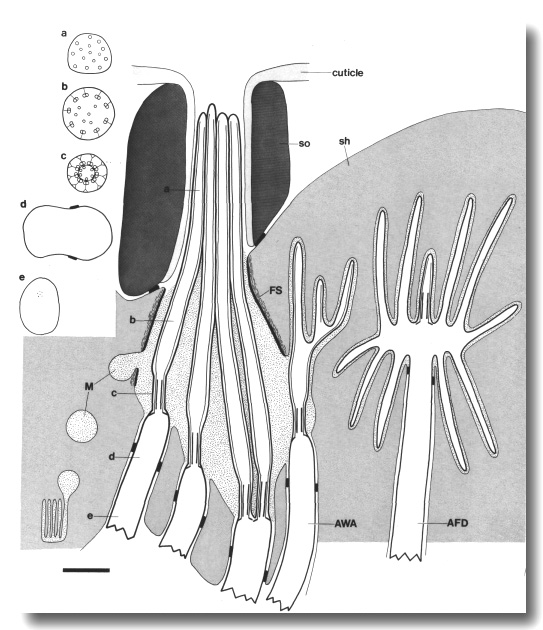
Figure 2. Schematic longitudinal section through amphid
sensillum in wild-type. The amphid channel is formed from a socket cell
(so) and a sheath cell (sh). The socket cell is joined by belt junctions to
surrounding hypodermal cells (not shown). The socket channel is lined with cuticle
that is continuous with the external cuticle. The anterior sheath channel has
a dark, noncuticular lining surrounded by a filamentous scaffold (FS). The sheath
and socket cells are joined together by belt junctions encircling the channel.
The space between the cilia in the posterior sheath channel is filled with a
dark matrix (M) that appears to be packaged into vesicles further posterior,
transported forward, and deposited around the cilia. The dendrites of three
channel neurons and one wing neuron (AWA)
are shown. The distal segment of the AWA
cilium leaves the fascicle of channel cilia to re-invaginate the sheath cell.
The AFD dendrite remains
separate from the fascicle of wing and channel cilia. All of the dendrites form
belt-shaped junctions with the sheath cell near their point of invagination.
The inset shows enlarged cross sections of a channel dendrite through the distal
segment (a), the middle segment (b), the transition zone (c), the neuron/sheath
junction (d), and the main dendrite in the papillary nerve (e). Main scale bar
is 1.0 micrometer
The phasmids, a pair of lateral sensilla in the tail, are similar
but smaller chemosensory organs (Sulston et al 1980; Hall and Russell 1986;
White et al, 1986). In newly hatched larvae, each phasmid comprises two ciliated
dendrites (PHA and PHB),
a sheath cell, and a socket cell. The neurons resemble the amphid channel neurons
in that their cilia extend into a socket channel that is open to the external
medium.
Below the cilia, the sensory dendrites are joined to the sheath
cell by belt junctions (Fig. 2). These have been described both as tight junctions
(Ward et al, 1975) and as desmosomes
(Ware et al, 1975) and may
have properties of both. Similar belt junctions encircle the channels, joining
the sheath and the socket cells together. Finally, belt junctions join the socket
cells to the surrounding hypodermis.
The channels of the amphid sheath and socket cells appear to originate
by different mechanisms (Wright, 1980). The sensory dendrites deeply invaginate
and, excepting the AFD neuron,
completely penetrate the sheath cell so that it is topologically a solid torus
with 11 holes. In contrast, the socket cell wraps around the receptor channel
and forms a typical intercellular belt junction where it meets with itself.
Thus topologically it has no hole.
The channel of the socket cell is lined with cuticle that is continuous with the external cuticle (Fig. 3a). The sheath cell channel is not lined with cuticle. Instead, the anterior sheath channel, in the region where the cilia draw together into a tight fascicle, has a characteristic dark lining (Fig. 3b). More posterior, nearer the bases of the cilia, the dark lining is interrupted by matrix-filled vesicles fusing with the lumen. The cytoplasm adjacent to the anterior sheath channel contains longitudinally aligned microtubules and intermediate filaments. These filaments may form a scaffold for the receptor channel (Wright, 1980). A much thinner scaffold, joined at its ends to the self-junction, wraps around the socket channel (Fig. 3a).
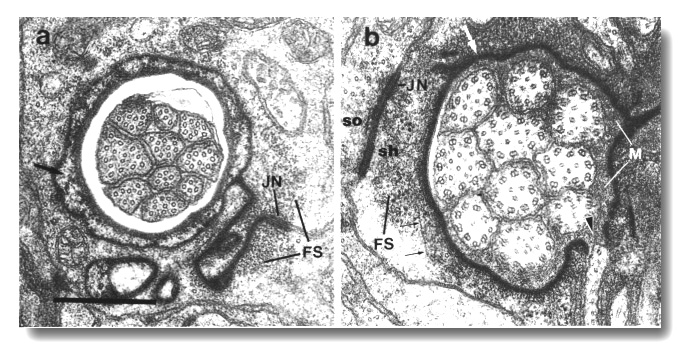
Figure 3. Amphid socket and sheath channels in wild-type,
(a) Section through amphid socket cell about 3.0 micrometer from the tip of
the head. The distal segments of the ten channel cilia are present. The cilia
contain both large (13 protofilament) and small (11 protofilament) diameter
microtubules. These are the A fibers of the nine doublet microtubules and the
inner singlet microtubules, respectively (Chalfie and Thomson, 1982). The socket
channel is lined by cuticle (black arrow). The self-junction (JN) and an associated
scaffold of intermediate filaments (FS) are also visible, (b) Section 2.5 micrometer
posterior to (a) through the amphid sheath cell showing the middle segments
of the channel cilia. The B subfibers of the doublets are complete. A variable
number of inner singlet microtubules are also present. Traces of matrix (M)
surround and separate the cilia at this level and more posteriorly. The channel
is lined by a dark material (white arrow) and the surrounding cytoplasm is filled
by a scaffold of longitudinal microtubules and intermediate filaments (FS).
A rare circumferential filament is seen in the plane of section (small black
arrows). Part of the belt junction (JN) between the sheath (sh) and socket (so)
cells is also visible. The dark lining and filament scaffold are interrupted
where the AWB cilium separates
from the main fascicle and invaginates the sheath cell (arrowhead). Scale bar
is 0.5 micrometer.
In glutaraldehyde fixed animals, a dark matrix surrounds the cilia
in the posterior sheath channel (Figure 4a). The matrix material appears to
be synthesized at lamellae posterior to the cilia and transported forward in
membrane-bound vesicles which later fuse with the channel lumen (Wright, 1980).
The matrix material of the amphid sheath cells, and a similar material in the
other sensilla, is not well preserved in animals fixed with Os04
alone. In consequence, several published reports erroneously describe an empty
space around the cilia or empty vesicles in the sheath cytoplasm. The matrix
material, though separating the cilia in the posterior channel, gradually thins
until the membranes of the channel cilia are in direct apposition in the anterior
sheath and the socket channel (Fig. 3). The pattern of fasciculation of the
channel cilia is invariant in wild-type animals (Ward
et al, 1975; Ware et al,
1975).
Description of Other Head Sensilla
In addition to the amphids, four classes of cuticular sensilla (cephalic, inner
labial, outer labial quadrant, and outer labial lateral) are found in the tip
of the head (Ward et al, 1975;
Ware et al, 1975). They
resemble the larger amphid sensilla in having two support cells, a sheath and
a socket, that form channels around the ciliated portion of the dendrites. They
differ from the amphids in that the socket channels are not lined with cuticle.
Most of the structural components of the amphid sensilla described above are
also found, reduced in size, in these sensilla.
The tip of the head has six symmetrically arranged lips (2 dorsal,
2 ventral, and 2 lateral). An inner labial sensillum is found on the apex of
each lip. These sensilla each contain two ciliated dendrites (IL1
and IL2) (Fig. 1). The dorsal and ventral
lips also contain a cephalic and an outer labial quadrant sensillum. The cephalic
sensilla have a single dendrite (CEP)
in hermaphrodites and an additional dendrite (CEM) in males. The outer labial
quadrant sensilla have a single dendrite (OLQ).
The lateral lips contain, in addition to an inner labial and an amphid sensillum,
an outer labial lateral sensillum. The outer labial lateral sensilla have a
single dendrite (OLL).
After passing through the socket channels, the IL1,
CEP, OLQ,
and OLL cilia end embedded
in the subcuticle and are believed to be mechanosensory. In contrast, the tips
of the IL2 and CEM cilia
completely penetrate the cuticle and are believed to be chemosensory.
Finally, two classes of ciliated dendrites (BAG
and FLP) found in the lateral
lips are not surrounded by support cells (Fig. 1). Their cilia end somewhat
behind the cuticle in bag and flap-shaped sheets, respectively, that envelop
short projections from the inner labial socket cells.
Ultrastructure of Amphid Cilia
The dendrites of amphid channel neurons ASE,
ASG, ASH,
ASI,
ASJ, and ASK each end with a single cilium
about 7.5 micrometer long in adults (Ward
et al, 1975; Ware et al,
1975). The dendrites of channel neurons ADF
and ADL are similar but each
ends in a pair of cilia (Figs. 2, 3). Three segments can be distinguished in
these cilia. The proximal segment, which corresponds to the transition zone
of the motile flagella in Chlamydomonas (Ringo, 1967), is a constriction
at the base of the cilium about 0.27 micrometer in diameter and up to 1.0 micrometer
in length. It comprises nine doublet-microtubules joined to the membrane by
Y-shaped links (Gilula and Satir, 1972) and drawn inward by attachments to a
central cylinder (Fig. 4b). A variable number of singlet microtubules are attached
to the inner surface of the cylinder. The central cylinder may correspond to
the apical rings found in the transition zones of cilia in some organisms. The
inner singlets in C. elegans differ from the central pair of microtubules
found in motile cilia in that they originate at the base of the transition zone
rather than above it. The inner singlets, like axonal microtubules in C.
elegans, have only 11 protofilaments whereas the A and B subfibers of the
peripheral doublets have 13 and 11 protofilaments, respectively (Chalfie and
Thomson, 1982).
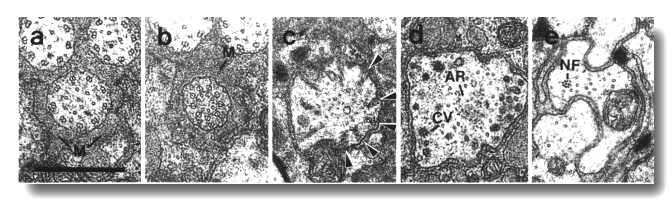
Figure 4. Amphid channel cilia in wild-type, (a) Section
through the middle segment of a channel cilium. Nine doublet microtubules are
attached to the membrane and seven smaller singlet microtubules occupy the center.
Matrix (M) separates the several cilia in the sheath channel. (b) Section 0.8
micrometer posterior to (a) through the transition zone. The nine doublets are
drawn together by the apical ring. The links are clearly Y shaped at their attachment
to the membrane. The seven singlets are attached to the inner face of the apical
ring. (c) Section 1.6 micrometer posterior to (a) through the transitional fibers
(arrowheads) that join the ends of the doublets radially to the cell membrane.
There is no basal body in the center of the dendrite but only an amorphous root.
(d) Section 2.5 micrometer posterior to (a). The dendrite is much larger in
diameter than at the cilium and is filled with coated pits and vesicles (CV).
The amorphous root (AR) may contain neurofilaments. (e) Section 6.6 micrometer
posterior to (a) and proximal to the neuron/sheath junction. The amorphous root
has gradually thinned to reveal a fascicle of ten neurofilaments (NF). Scale
bar is 0.5 micrometer.
The middle segment differs from the transition zone in lacking the central
cylinder. The doublets, still linked to the membrane, spread apart somewhat and
the cilium flares in diameter (Fig. 4a). The Y-shaped bases of the membrane
links are no longer apparent, perhaps relaxing against the membrane in the
absence of inward tension on the doublets. The inner singlet microtubules
continue, unattached, in the center of the cilium. The middle segment of the
channel cilia corresponds to the flagellar shaft in Chlamydomonas and continues
for about 4 micrometer.
The B subfibers of the doublet microtubules are gradually lost near the end of
the middle segment (Fig. 3b). The distal segment, about 2.5 micrometer long,
contains only A subfibers and inner singlet microtubules (Fig. 3a). The membrane
links are probably also lost. The distal segment, roughly the portion in the
socket channel, may be the transducing region of the cilium.
The amphid cilia, like sensory cilia in nematodes generally have no apparent
basal bodies (Wright, 1980). The cilia terminate proximally in connections from
the peripheral doublets to the cell membrane (Fig. 4c; see also Figs. 5g, 7c).
These terminal connections may be equivalent to the transitional fibers seen in
other organisms (Reese, 1965; Ringo, 1967). As they have complex substructure,
they conceivably also contain some residue of the nematode centriole.
Unlike the channel cilia which are all cylindrical, each of the
amphid wing cilia (AWA, AWB,
and AWC) has a unique shape
(Ward et al, 1975; Ware
et al, 1975). The AWC
cilium spreads vertically into two enormous sheets, resembling wings. These
wings and the surrounding sheath cell, fill much of the left and right hemisectors
at the tip of the animal (Fig. 1). The AWA
and AWB cilia are smaller
than AWC and comparable in
size to the channel cilia. The distal segments of the AWA
cilia split into several small projections each containing one or more of the
original nine doublet microtubules (Fig. 2). The AWB
dendrite, like ADF and ADL,
ends in a pair of cilia. The distal segments of the AWB
cilia do not split like the AWA
cilia but are somewhat flattened and irregular.
None of the amphid dendrites contain striated ciliary rootlets.
Instead, an amorphous gray material extends posteriorly from the centers of
the channel and wing cilia for about a micrometer (Fig. 4d). This material gradually
thins, revealing a fascicle of 3 to 12 neurofilaments that continue at least
several micrometers further (Fig. 4e). It is likely that these neurofilaments
are actually embedded in the amorphous root and extend to the base of the cilia.
The amorphous root is reduced or absent in the AFD
dendrites. In those cells, a fascicle of neurofilaments extends to the base
of the cilia. Finally, numerous coated pits and vesicles are found in all the
amphid dendrites just proximal to the cilia (Fig. 4d).
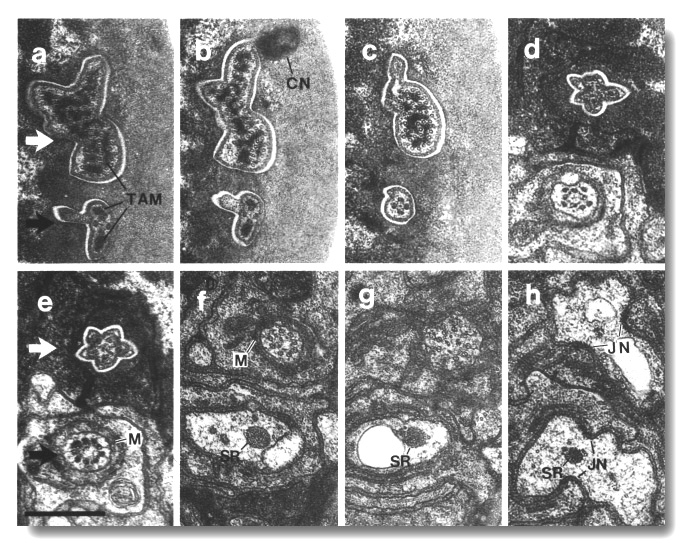
Figure 5. CEP
and OLQ cilia in wild-type,
(a) Section through the distal segments of CEP
(white arrow) and OLQ (black
arrow) cilia in wild type. The CEP
cilium is filled with microtubules interspersed with an amorphous dark tubule-associated
material (TAM). The outermost microtubules appear to have fine attachments to
the membrane. The OLQ cilium
contains four doublet microtubules joined together into a square by thick cross-bridges.
The corners of the square point radially and circumferentially. Inside the square,
fine radial arms connect the doublets to a small hub. Small lumps of dark material
flank the circumferential doublets. This tubule-associated material (TAM) may
also be attached to the membrane. (b) Section 0.15 micrometer posterior to (a)
showing the end of the cuticle-associated nubbin (CN) of the CEP
cilium. The OLQ cilium has
a similar nubbin about 1 micrometer more anterior. (c) Section 0.6 micrometer
posterior to (a). The supernumerary microtubules and the dark tubule-associated
material of the CEP cilium
are reduced. The tubule-associated material of the OLQ
cilium is no longer present. (d) Section 2.0 micrometer posterior to (a) through
the middle segment of the CEP
cilium. No supernumerary microtubules or tubule-associated material are present.
Nine doublet microtubules are present in the OLQ
cilium, four of which are joined by cross-bridges. The A and B subfibers of
most of the microtubules appear filled. The A subfibers of three doublet microtubules
in the square appear empty, (e) Section 2.1 micrometer posterior to (a) through
the transition zone of the OLQ
cilium. All nine doublet microtubules are attached to the membrane by Y-shaped
links. Matrix (M) surrounds the cilium. (f) Section 3.3 micrometer posterior
to (a) through the transition zone of the CEP
cilium. The doublet microtubules are attached to the membrane by Y-shaped links.
In contrast to the OLQ cilium,
the A and B subfibers of the CEP
cilium appear empty. Matrix (M) surrounds the cilium. A large striated ciliary
rootlet (SR) is present in the OLQ
dendrite. (g) Section 3.8 micrometer posterior to (a) through the transitional
fibers of the CEP cilium.
(h) Section 4.5 micrometer posterior to (a) through neuron/sheath junctions
(JN). The CEP dendrite has
no prominent rootlet. Scale bar is 0.5 micrometer.
Ultrastructure and Specialization of Mechanocilia
The transition zones of the various mechanocilia resemble those of the amphid
cilia. In particular, central structures, probably short cylinders, join the
inner faces of the doublets. In many of the mechanocilia, some peripheral doublets
terminate just distal to the transition zone. In the CEP
and OLL cilia, for example,
usually only five membrane-linked doublets continue in the middle segments (Figs.
5e,6). The distal segments of the CEP
and OLL cilia contain an
amorphous dark material and associated microtubules common to proved mechanocilia
in insects (Ward et al, 1975;
Ware et al, 1975; Thurm
et al, 1983). In the CEP
cilia, the microtubules are interspersed with the dark material and mold it
into irregular rods (Fig. 5a). In the OLL
cilia, the dark material is not interspersed with microtubules but forms a large
aggregate surrounded by a single layer of microtubules. In both the CEP
and OLL cilia, the outermost
microtubules appear to have fine attachments to the membrane. The microtubules
in the distal segments are all singlets and, at least a majority, are supernumerary
in that they do not derive from the nine-doublet microtubules of the axoneme
nor are they central singlet microtubules arising at the apical ring as in the
amphid cilia. The supernumerary microtubules and the dark tubule-associated
material are confined to the region embedded in the cuticle (Fig. 6).
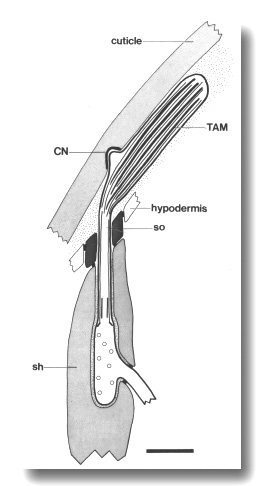
Figure 6. Schematic of longitudinal section through the CEP
sensillum in wild-type showing the receptor channel formed by the sheath (sh),
socket (so), and hypodermis. The distal segment, containing supernumerary microtubules
and dark tubule-associated material (TAM), is embedded in the subcuticle. A
small nubbin (CN) extends into the cuticle near the base of the distal segment.
Coated vesicles (circles) are present in the CEP
dendrite proximal to the cilium and distal to the neuron/sheath junction. Scale
bar is 1.0 micrometer.
The OLQ cilia
are unique in two respects. First, the A and B subfibers have filled cores giving
the doublets an exceptionally dark appearance. Second, exactly four of the nine
doublets extend through the cilium (Figs. 5a-e). These four doublets are not
membrane linked but are joined along their lengths by thick cross-bridges to
form a square. Fine radial arms join these doublets to a small hub in the center
of the square. The corners of the square always point radially or circumferentially
in the wild type. In the distal segment, embedded in the subcuticle, one or
two small aggregates of amorphous dark material, resembling the tubule-associated
material of the CEP and OLL
cilia, flank the doublet microtubules at the circumferential corners, but not
the radial corners (Figs. 5a,b). This material may also be connected to the
membrane.
The tips of the IL1
cilia contain a disc of dark material attached on both faces to the ciliary
membrane (Fig. 7a). This dark material is positioned in the cuticle in such
a way as to be compressed by outward radial deflections of the papillary protrusions
caused by head-on contact with external objects.
The distal segments of the CEP,
OLL, and OLQ
cilia are anchored in cuticle by a small dark nubbin (Ward
et al, 1975; Ware et al,
1975). In the CEP and
OLQ neurons the nubbin occurs
at the base of the transducing region (Figs. 5b, 6). The OLL
cilia differ in that the nubbin is at the distal tip of the cilium and the supernumerary
microtubules and tubule-associated material are proximal to the nubbin.
Finally, three classes of sensory cilia (BAG,
IL1, and OLQ)
in the hermaphrodite have large striated rootlets (Ward
et al, 1975; Ware et al,
1975). The rootlets extend into the center of the transition zone (Figs.
7b,c).
Fewer than nine peripheral doublets have been reported for some
classes of cilia in C. elegans (Ward
et al, 1975; Ware et al,
1975). Using glutaraldehyde-fixed adults, we consistently found nine doublets
in the transition zone of the BAG,
CEP, IL1,
and OLQ cilia. Since not
all nine doublets extend into the shaft in some of these classes, they could
be overlooked in a coarse series. All the IL2
cilia examined in wild-type adults have fewer than nine doublets in the shaft
and no well-formed transition zone.
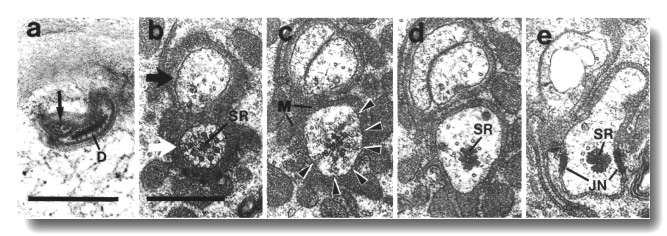
Figure 7. IL1
and IL2 cilia in wild-type.
(a) Section through the dark membrane-attached disc (D) at the tip of the IL1
cilium. The small IL2 cilium
(black arrow) continues anterior to a small opening in the cuticle. The IL1
disc is positioned in the cuticle in such a way as to be compressed by head-on
collisions of the animal, (b) Section through the transition zone of an IL1
neuron (white arrow). A striated ciliary rootlet (SR) extends into the center
of the cilium. The dendrite of an IL2
neuron (black arrow) shares the sensillum. (c) Section 0.15 micron posterior
to (b) showing transitional fibers (arrowheads) in the IL1
cilium. Note the increase in diameter of the cilium at this point. Matrix-filled
vesicles (M) in the sheath cytoplasm, (d) Section 0.3 micrometer posterior to
(b) showing the striated rootlet (SR). (e) Section 0.8 micrometer posterior
to (b) showing the IL1/sheath
junction (JN). The striated rootlet (SR) continues for about 9 micrometer. Scale
bars are 0.5 micrometer.
Mechanism of Dye Filling
When living C. elegans are placed in solutions of 5-fluorescein isothiocyanate (FITC),
six pairs of neurons in the head and two pairs in the tail fill with dye (Fig.
8a). Their cell bodies and processes become visible within 5 min and reach a
maximum brightness within about 2 hr when stained in 0.1 mg/ml FITC. Dye filling
proceeds equally well at 0° as at 20°. Once filled with 5-fluorescein
isothiocyanate, the neurons remain brightly stained for many hours in the
absence of dye. Staining with fluorescein, in contrast, reverses completely in
the course of an hour. Presumably, 5-fluorescein isothiocyanate, but not free
fluorescein, can combine with amino groups within the cell and become either
immobile or impermeant to cell membranes. In support of this, 5-fluorescein isothiocyanate, when coupled to bovine serum albumin, cannot enter the neurons
from the outside.
We tested a variety of other fluorescent dyes and none, except certain
fluorescein derivatives, accumulate in the amphid and phasmid neurons. The
fluorescein derivatives that stain the neurons are weak acids and exist as both
neutral and anionic forms within the physiological range of pH values. In their
uncharged forms, favored by lower pH, they can probably diffuse across cell
membranes.
The FITC-filled neurons in the head and tail were identified as
amphid channel neurons (ADF,
ASH, ASI,
ASJ, ASK,
and ADL) and phasmid channel
neurons (PHA and PHB),
respectively (Hedgecock et al, 1985).These cells stain in larvae of all stages
and in adults. To learn whether fluorescein enters these neurons through their
exposed sensory cilia, we killed the phasmid support cells in newly hatched
larvae using a laser microbeam (Sulston and White, 1980). These animals were
tested as adults for dye uptake into the phasmid neurons. Killing the socket
cell (2 animals), which presumably disconnects the sheath and cilia from the
cuticle, or the sheath cell (1 animal) abolished filling of the ipsilateral
neurons without affecting the neurons of the contralateral phasmid sensillum.
Control ablations of neighboring cells did not affect dye uptake.
The amphid channel neurons ASE
and ASG, the IL2
neurons, and the various male-specific chemosensory neurons do not appear to
fill with fluorescein dyes. Thus access of the sensory dendrites to the dye
is apparently necessary but not sufficient to ensure filling. Apparently a physiological
property, shared by some but not all sensory neurons, is also required for filling.
A simple suggestion is that for dye to fill the entire neuron, the rate of dye
entry through the sensory receptor must be greater than the rate of dye leakage
into the body cavity from the sensory process. The rate of entry is controlled
by the geometry, and possibly, membrane properties of the exposed dendrites.
The rate of leakage from the processes might depend on membrane potential or
intra-cellular pH.
Identification of Behavioral Mutants with Impaired FITC Uptake
Mutants with sensory defects have been isolated by selections involving chemotaxis
toward Na+ or Cl- ions (tax and che genes: Dusenbery et al, 1975;
Lewis and Hodgkin, 1977), thermotaxis (ttx genes: Hedgecock and
Russell, 1975), male mating (Lewis and Hodgkin, 1977, Hodgkin, 1983), avoidance
of solutions of high osmotic strength (osm genes: Culotti and Russell, 1978),
dauer larva formation (daf genes: Riddle et al, 1981), coarse mechanical
stimulation (mec genes: Chalfie and Sulston, 1981), egg-laying (egl
genes: Trent et al, 1983), and formaldehyde-induced fluorescence (FIF) to visualize
catecholamine (dopamine) containing mechanosensory neurons (CEP,
ADE, and PDE)
(cat genes: Sulston et al, 1975).
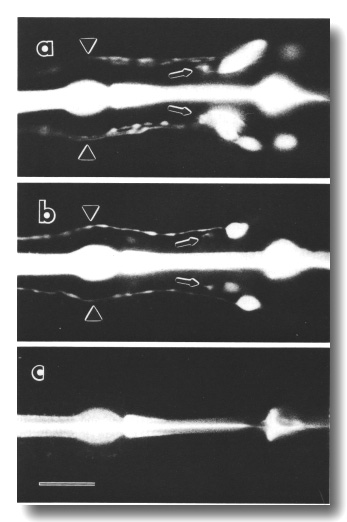
Figure 8. FITC uptake by amphid neurons in living animals,
(a) Ventral view of the wild type animal. Six cells on each side, not all resolved
in this focal plane, are filled with dye (Hedgecock et al, 1985). Processes
from the sensory cilia (arrowheads) and processes to the nerve ring neuropil
(arrows) are also visible, (b) Ventral view of che-10 (e1809)
mutant. One cell on each side is brightly stained in this individual. A second
cell is faintly stained on the right side, (c) Ventral view of che-10
(e1809) mutant. No cells are stained in this individual. The bright central
stripe is fluorescence from dye bound to the sclerotized cuticle lining the
pharynx. Scale bar is 20 micrometer.
We examined alleles of all the published cat, che, daf, mec,
osm, tax, and ttx genes for defects in FITC uptake into chemosensory neurons.
All of the cat, ttx, and mec mutants, with the exceptions of mec-1
and mec-8, were essentially normal in dye filling. In contrast, all of
the osm mutants and some of the che, daf, and tax mutants
are defective in dye uptake, affecting both the amphid and phasmid neurons (Fig.
9, Table 1).

Figure 9. Genetic map. Map positions of genes affecting FITC
uptake are shown below the lines. Marker genes are shown above the lines. The
positions are based on the data of Lewis and Hodgkin (1977), Culotti and Russell
(1978), Riddle et al (1981), Rand and Russell (1984), R. Herman (1984), and
new data, listed below, obtained using the dye-uptake phenotypes of the mutants.
Two-factor distances, obtained by scoring the DPY, UNC, or LON progeny of cis-
linked heterozygotes, are expressed as the number of recombinant chromosomes
to total chromosomes examined. No corrections are made for multiple events.
Three-factor gene orders and distances are shown in the format of the map database
maintained by the Caenorhabditis Genetics Center (see Swanson et al, 1984).
We tested whether any of these mutations, isolated in different
laboratories, fail to complement. Indeed, the mutations che-3 (el 124), che-8
(e1253), and osm-2 (p801) on linkage group I all fail to complement
for FITC uptake. Similarly, mutations daf-10 (el387) and osm-4 (p821)
on linkage group IV represent a single gene. Finally, the unmapped tax
mutation, a83 (formerly RS3, Dusenbery et al, 1975) is an allele of osm-1.
We also isolated nine new mutants with reduced dye uptake. These
fall in two of the known osm genes and five new genes designated che-10
through che-14. Excluding the mec-1 and mec-8 alleles,
there are now 25 mutations, defining 14 complementation groups, which reduce
or eliminate FITC uptake by amphid and phasmid neurons (Table 1, Fig. 9). A
spectrum of behaviors was tested for each mutant (Table 1).
Dye Filling of Mutant Mechanosensory Neurons
Mechanosensory neurons do not normally fill with FITC. In some chemosensory
mutants, however, certain mechanosensory neurons, including CEP,
ADE, and
PDE neurons, occasionally stain brightly (Table 1). In many of the mutants
showing occasional staining of mechanosensory neurons in hermaphrodites, occasional
ray neurons also stain in males (Table 1). We examined ray staining in detail
in osm-1 (p808) males. It appears that neurons from each of the 18 ray
sensilla are capable of staining. Apparently only one neuron per sensillum can
fill with dye. We speculate that the stained cells are RnA neurons, rather than
RnB neurons, as the RnB dendrites are externally exposed, yet nonstaining, in
wild-type males (Sulston et al,
1980).
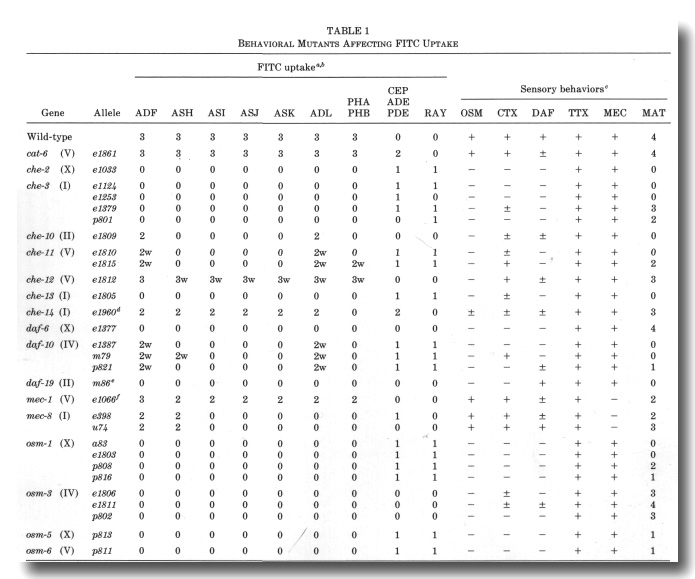
a The following mutants were found to have normal FITC
uptake: che-5 (e1073), che-6 (e1126), che-7 (ell28), daf-1 (e1287), daf-2
(e1370), daf-3 (e1376), daf-4 (e1364), daf-5 (e1386), daf-7 (el372), daf-8 (e1393},
daf-9 (e1406), daf-11 (m47), daf-12 (m20), daf-13 (m66), daf-14 (m77), daf-15
(m81), daf-16 (m26), daf-17 (m27), daf-18 (e1375), and daf-20 (m25). Heat-sensitive
alleles were tested at nonpermissive temperature (25°). In che-1 (e1034)
mutants, an additional class of amphid neurons often stains.
b The frequency and intensity of staining of neurons is indicated qualitatively:
3, usually or always stains; 2, frequently stains; 1, occasionally stains; 0,
rarely or never stains. A suffix w indicates that the staining intensity is much
weaker than in wild-type.
c Avoidance of concentrated NaCl (osmotic, OSM) was tested with a population
assay (Culotti and Russell, 1978). Attraction (chemotaxis, CTX) was tested individually
using dilute gradients of NaCl (Ward, 1973). Dauer larva formation (DAF) was
tested on crowded, starved plates using sodium dodecyl sulfate to kill nondauer
larva (Cassada and Russell, 1975). The cuticles of survivors were examined using
Nomarski optics to confirm the presence of dauer-specific alae. Ability to follow
isotherms (thermotaxis, TTX) was tested individually in radial temperature gradients
(Hedgecock and Russell, 1975). Touch sensitivity (mechanosensory, MEC) was tested
with an eyebrow hair (Chalfie and Sulston, 1981). Males were obtained from him-5
(e1490) double mutants and their mating ability (MAT) was tested by the
procedure of Hodgkin (1983). All behaviors, except mating, were scored either
(-) no response, (±) intermediate response, or (+) essentially wild-type response.
Male mating ability was scored according to Hodgkin (1983): 4, very efficient
mating (30-100% of wild-type efficiency); 3, efficient mating (10-30% of wild-type);
2, poor mating (1-10% of wild-type); 1, very poor mating (less than 1% of wild-type);
and 0, no detected matings.
d For each amphid sensillum in che-14 (e1960), either all six
neurons stain or none stain. In addition to the CEP
neurons, unidentified sensory neurons with cell bodies anterior to the nerve
ring frequently stain in che-14 (e1960). In the OSM assay, about 10%
of the che-14 (e1960) animals failed to avoid concentrated NaCl.
e The daf-19 (m86hs) mutants form dauer larvae constitutively,
particularly at high temperature (D. Riddle, personal communication). There
is no FITC staining at either permissive (15°, adults and dauers) or nonpermissive
temperature. (25°, dauers only).
f The phasmid neurons were examined in forty mec-1 (e1066) mutants.
Both neurons stained brightly in 58 sensilla, only one neuron stained in 15
sensilla, and no neurons stained in 7 sensilla. In comparison, both phasmid
neurons stained in 78 sensilla and no neurons stained in 2 sensilla in 40 wild-types.
Mutants of two genes, cat-6 and che-14, show a much
higher frequency of dye filling by mechanosensory neurons. In cat-6 mutants,
the amphid and phasmid neurons stain normally, but the CEP,
ADE, and PDE
neurons also stain brightly in many animals. The proportion of these mechanosensory
neurons staining is greatest just after molts (Fig. 10). In che-14 mutants,
the phasmid neurons never stain and the amphid neurons frequently fail to stain
(Table 1). The CEP, ADE,
and PDE neurons stain brightly
in many animals as do additional, unidentified sensory neurons in the head.
As shown below, the CEP dendrites,
and presumably the other classes that stain, have abnormal access to the external
medium in cat-6 and che-14 mutants.
Mutants of two genes, cat-6 and che-14, show a much
higher frequency of dye filling by mechanosensory neurons. In cat-6 mutants,
the amphid and phasmid neurons stain normally, but the CEP,
ADE, and PDE
neurons also stain brightly in many animals. The proportion of these mechanosensory
neurons staining is greatest just after molts (Fig. 10). In che-14 mutants,
the phasmid neurons never stain and the amphid neurons frequently fail to stain
(Table 1). The CEP, ADE,
and PDE neurons stain brightly
in many animals as do additional, unidentified sensory neurons in the head.
As shown below, the CEP dendrites,
and presumably the other classes that stain, have abnormal access to the external
medium in cat-6 and che-14 mutants.
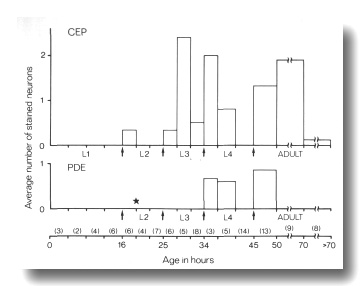
Figure 10. FITC Uptake by CEP
and PDE neurons in cat-6
(e1861) mutants. Animals were stained with FITC for 2 hr and then examined
by fluorescence microscopy for uptake into CEP
and PDE neurons and by Nomarski
microscopy to determine their approximate age. The average number of stained
neurons per animal is shown as a function of age. Arrows mark the four larval
molts. The star indicates the time of birth of the PDE
neurons (Sulston and Horvitz, 1977). Numbers in parentheses indicate how many
animals in each age group were examined. Each animal has a total of four CEP
neurons and two PDE neurons
(White et al, 1986).
Mutants with Short Axonemes in all Classes of Cilia
Mutations in three genes, che-13 (e1805), osm-1 (p808), and osm-5
(p813), shorten the axonemes of all classes of sensory cilia in the head.
Singlet or doublet microtubules, joined to the membrane by Y links, assemble
below the transition zones. The various distal specializations of the mechanocilia
also assemble ectopically in these mutants.
The peripheral doublets of the amphid channel cilia end within
about 2 micrometer of the transition zone (Fig. 11). The inner singlets do not
extend beyond the apical ring. The wing cilia are similarly affected. Interestingly,
the AWC cilium fails to spread
into sheets and the surrounding sheath cell is correspondingly reduced. The
AFD cilia, although fairly
short in wild-type, are reduced further and often tilted. The AFD fingers themselves
are unaffected in number or appearance.
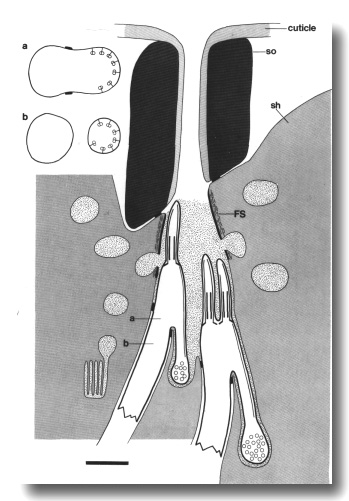
Figure 11. Schematic of amphid sensillum in osm-1 (p808).
The amphid cilia are extremely short. Doublet microtubules attached to the membrane
by Y links, assemble ectopically below the transition zone. These membrane-linked
doublets, like the normal cilia, are topologically distal to the neuron sheath
junction. They create cilia-like posterior projections that terminate in vesicle-filled
swellings. Abnormal large matrix-filled vesicles accumulate in the sheath cell.
Insets show cross sections through the level of the neuron/sheath junction (a)
and through the ectopic posterior projection (b). Scale bar is 1.0 micrometer.
Doublet microtubules, joined to the membrane by Y links, assemble below the
cilia in these mutants. These doublets are not continuous with the nine
peripheral doublets of the cilium (Fig. 12a). The ectopic doublets do not
generally cross the neuron/sheath junctions but instead create a posterior
projection within the sheath cell (Fig. 12b). Like normal cilia, these
projections are topologically distal to the junctions. They strikingly mimic the
middle segment of a normal cilium (Fig. 12c). They end blindly within the sheath
cell and are usually filled with vesicles where they terminate (Fig. 12d). The
occasional doublets that cross the neuron/sheath junction, lose their membrane
links below the junction.
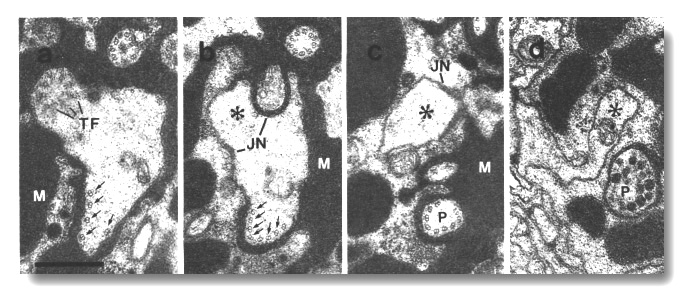
Figure 12. Amphid cilia in osm-5 (p813) mutant, (a)
Section through an ADF dendrite.
The transitional fibers (TF) of one cilium are visible in the upper left. The
transition zone of the second cilium is 0.5 micrometer distal to this section
in the upper right. In the lower part, ectopic doublet microtubules are attached
to the membrane by Y links (arrows). Matrix material (M) surrounds the dendrites.
(b) Section 0.8 micrometer posterior to (a) showing the main dendrite (star)
leaving the sheath cell. The ectopic doublets (arrows) segregate into a posterior
projection that, like a normal cilium, is topologically distal to the neuron/sheath
junction (JN). (c) Section 1.2 micrometer posterior to (a). The ectopic projection
(P) is completely separated from the main dendrite (star). Except for the absence
of inner singlet microtubules, the projection strikingly resembles the middle
segment of a normal cilium. (d) Section 3.0 micrometer posterior to (a). The
ectopic doublets have terminated and the ectopic projection (P) terminates in
a vesicle-filled swelling within the sheath cell. The main dendrite (star) continues
toward the neuron cell body. Scale bar is 0.5 micrometer.
As judged by the hooks on microtubules with partial B subfibers, the ectopic
doublets have the opposite clocksense to the nine ciliary doublets in adjacent
sections. As the ectopic tubules project posteriorly and the cilia project
anteriorly, both classes of doublets have the same relative clocksense. In
particular, the B subfibers are counterclockwise of their respective A fibers
for a viewer looking from proximal to distal.
The amphid sheath channel in these mutants contains more matrix than wild-type
and much of the space normally occupied by cilia is filled with matrix instead.
Abnormal large matrix-filled vesicles accumulate in the anterior cytoplasm of
the sheath cell. Often these vesicles are partially fused with the channel.
The CEP, IL1,
IL2, OLL, and OLQ
axonemes are greatly reduced in length in che-13 (e1805), osm-1 (p808),
and osm-5 (p813) mutants (Fig. 13). The dendrites themselves, however,
continue and may reach the cuticle. In particular, the CEP,
OLL and OLQ
dendrites form cuticle-attached nubbins. Empty tunnels in the subcuticle are
found anterior to CEP and,
less often, the OLQ dendrites
suggesting that these dendrites once extended somewhat further but have retracted,
usually to the nubbin.
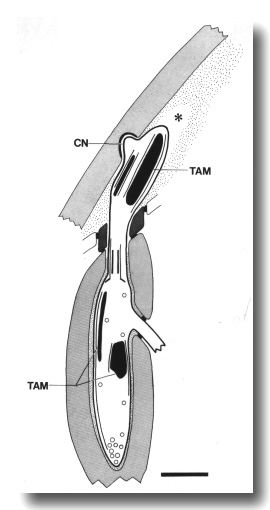
Figure 13. Schematic longitudinal section through the CEP
cilium in osm-5 (p813) mutant. The cilium is truncated distal to the
transition zone. Normal rod-shaped and large, ball-shaped aggregates of tubule-associated
material (TAM) and supernumerary microtubules assemble both distal and proximal
to the cilium. The dendrite forms a normal cuticle-attached nubbin (CN). An
empty tunnel (star) in the subcuticle suggests that the distal dendrite has
retracted. Scale bar is 1 micrometer.
The transition zones of the CEP,
IL1,
IL2, OLL, and OLQ
cilia, although normal in structure, are frequently mispositioned along the
dendrite either anteriorly, to the level of the socket channel or beyond (Fig.
14a), or posteriorly, to the level of the neuron/sheath junction (Fig. 16a)
or even into the ectopic posterior projections. As in the amphid cilia, membrane-linked
microtubules assemble ectopically behind the cilia. These microtubules are generally
fewer and shorter than in the amphid cilia and are more often singlets than
doublets. Again, these ectopic membrane-linked microtubules do not cross the
neuron/sheath junction but instead create a posterior projection within the
sheath cell.
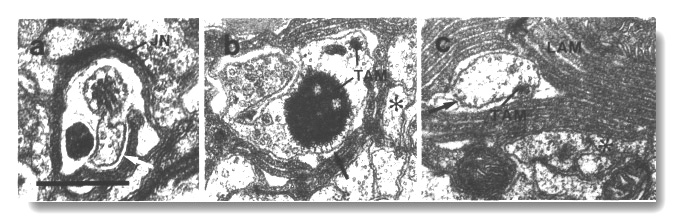 Figure 14. CEP cilia in osm-1 (p808). (a) Section
through the transition zone of a CEP
cilium. The axoneme is abnormally short and the transition zone is displaced
forward to the level of the sheath/socket junction (JN). Excess dendritic membrane
is drawn aside from the cilium (white arrow). (b) Section 2.7 micrometer posterior
to (a). The main CEP dendrite
(star) has passed out of the sheath cell. An ectopic posterior branch remains
within the sheath cell. It contains a small rod and an abnormal large aggregate
of dark tubule-associated material (TAM). Some of the microtubules surrounding
the dark material appear to be attached to the membrane (black arrow). (c) Section
5.1 micrometer posterior to (a) showing the main dendrite (star) of the CEP
neuron and an ectopic branch containing membrane-attached microtubules (black
arrow) and a rod of dark tubule-associated material (TAM). Lamellae (LAM) in
the sheath cell surround the ectopic branch. Scale bar is 0.5 micrometer.
Figure 14. CEP cilia in osm-1 (p808). (a) Section
through the transition zone of a CEP
cilium. The axoneme is abnormally short and the transition zone is displaced
forward to the level of the sheath/socket junction (JN). Excess dendritic membrane
is drawn aside from the cilium (white arrow). (b) Section 2.7 micrometer posterior
to (a). The main CEP dendrite
(star) has passed out of the sheath cell. An ectopic posterior branch remains
within the sheath cell. It contains a small rod and an abnormal large aggregate
of dark tubule-associated material (TAM). Some of the microtubules surrounding
the dark material appear to be attached to the membrane (black arrow). (c) Section
5.1 micrometer posterior to (a) showing the main dendrite (star) of the CEP
neuron and an ectopic branch containing membrane-attached microtubules (black
arrow) and a rod of dark tubule-associated material (TAM). Lamellae (LAM) in
the sheath cell surround the ectopic branch. Scale bar is 0.5 micrometer.
The supernumerary microtubules and associated dark material normally
found in the distal segments of the CEP
and OLL cilia were present
but positioned irregularly along the dendrites, both distal and proximal to
the residual cilia. Large, ball-shaped aggregates of the tubule-associated material
were often found in the ectopic posterior projections of the CEP
cilia (Fig. 14b).
The joined square of doublets is formed in the OLQ
cilia but generally fails to extend past the sheath channel. In many cilia,
the corners of the square do not point radially and circumferentially. In a
few cases, five rather than four doublets were joined by cross-bridges to make
an irregular pentagon with two central hubs (Fig. 15).
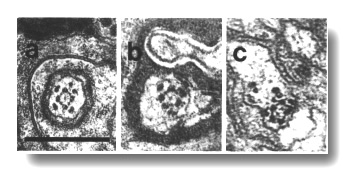 Figure 15. OLQ
cilia in wild-type, osm-5, and che-13 mutants. (a) Section through
wild-type OLQ cilium showing
nine doublet microtubules plus the cross-bridges that join four of them into
a central square. Inside the square, fine radial arms join the doublet microtubules
to a hub. (b) Section through osm-5 (p813) OLQ
cilium. Cross-bridges join five of the doublet microtubules into an irregular
pentagon. (c) Section through che-13 (e1805) OLQ
cilium. Cross-bridges join five of the doublet microtubules into an irregular
pentagon. Fine radial arms connect the doublet-microtubules to two separate
hubs. Scale bar is 0.5 micrometer.
Figure 15. OLQ
cilia in wild-type, osm-5, and che-13 mutants. (a) Section through
wild-type OLQ cilium showing
nine doublet microtubules plus the cross-bridges that join four of them into
a central square. Inside the square, fine radial arms join the doublet microtubules
to a hub. (b) Section through osm-5 (p813) OLQ
cilium. Cross-bridges join five of the doublet microtubules into an irregular
pentagon. (c) Section through che-13 (e1805) OLQ
cilium. Cross-bridges join five of the doublet microtubules into an irregular
pentagon. Fine radial arms connect the doublet-microtubules to two separate
hubs. Scale bar is 0.5 micrometer.
The dark material that normally flanks the circumferential corners was
fragmented and mispositioned.
The dark membrane-attached discs normally found at the tips of
the IL1 cilia were present
but displaced posteriorly in these mutants, often to the level of the transition
zone (Fig. 16a).
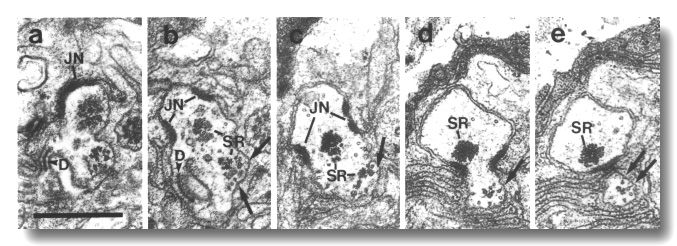 Figure 16. IL1 cilium in osm-1 (p808) mutant, (a)
Section through transitional fibers of an IL1
cilium. The cilium is displaced posteriorly from its wild-type position and
is nearly at the level of the IL1/sheath
junction (JN). The dark membrane-attached disc (D), normally present at the
distal tip of the IL1 cilium,
is also mispositioned. (b) Section 0.15 micrometer posterior to (a). Ectopic
membrane-attached singlet and doublet microtubules (arrows) extend posteriorly.
A large striated rootlet (SR) is associated with the cilium while a smaller
rootlet is recruited by the ectopic membrane-attached microtubules. (c-e) Sections
0.45, 1.1, and 1.2 micrometer posterior to (a). The ectopic microtubules (arrows)
and their associated rootlet segregate from the main dendrite and form a posterior
projection within the sheath cell. The main dendrite, and the large striated
rootlet (SR), leave the sheath cell. Scale bar is 0.5 micrometer.
Figure 16. IL1 cilium in osm-1 (p808) mutant, (a)
Section through transitional fibers of an IL1
cilium. The cilium is displaced posteriorly from its wild-type position and
is nearly at the level of the IL1/sheath
junction (JN). The dark membrane-attached disc (D), normally present at the
distal tip of the IL1 cilium,
is also mispositioned. (b) Section 0.15 micrometer posterior to (a). Ectopic
membrane-attached singlet and doublet microtubules (arrows) extend posteriorly.
A large striated rootlet (SR) is associated with the cilium while a smaller
rootlet is recruited by the ectopic membrane-attached microtubules. (c-e) Sections
0.45, 1.1, and 1.2 micrometer posterior to (a). The ectopic microtubules (arrows)
and their associated rootlet segregate from the main dendrite and form a posterior
projection within the sheath cell. The main dendrite, and the large striated
rootlet (SR), leave the sheath cell. Scale bar is 0.5 micrometer.
The striated ciliary rootlets of the IL1,
OLQ, and BAG
neurons are normal in these mutants and attach properly to the transition zone.
Interesting, the ectopic membrane-attached microtubules found in these mutants
also recruit small rootlets (Figs. 16b, c).
In an unexpected contrast to wild-type, well-formed transition
zones comprising a tight ring of nine Y-linked doublet microtubules were found
in all classes of cilia, including IL2,
in che-13, osm-1, and osm-5 mutants.
The osm-6 (p811) mutant has a similar, though perhaps less
severe, ultrastructural phenotype than the che-13, osm-1, and osm-5
mutants. The microtubules of the various classes of cilia extend further than
in the other mutants but ectopic membrane-attached microtubules still assemble
proximal to the cilia. The large wings of the AWC
cilia are reduced but not eliminated. The transition zones of the mechanocilia
in osm-6 (p811), in contrast to the other three mutants, are positioned
normally along the dendrites. The dark discs in the IL1
dendrites are also positioned normally at the tips but another mechanosensory
specialization, the supernumerary microtubules and dark tubule-associated material
of the CEP dendrites, assembles
ectopically. Possibly significant, the amphid sheath cytoplasm contains an excess
of small, unfused matrix-filled vesicles rather than the large vesicles found
in the other mutants. The osm-6 (p811) vesicles resemble the unfused
matrix-filled vesicles found in wild type except for their greater numbers.
daf-19 Mutants Lack All Classes of Cilia
The sensory dendrites in daf-19 (m86) mutants entirely lack cilia including
the transition zones. Vestigial centrioles, without membrane attachments, are
found in a few of the amphid dendrites (Fig. 17). No ectopic membrane-linked
microtubules are found in the amphid dendrites. A few membrane-associated singlet
microtubules are found in the CEP,
IL1, and OLQ
cilia. The amphid dendrites, and most of the mechanosensory dendrites, terminate
in club-shaped endings after invaginating, and forming belt-shaped junctions
with their respective sheath cells. The CEP
dendrites, though not the OLL,
and OLQ dendrites, extend
through their socket channels to end in cuticle-attached nubbins. Supernumerary
microtubules and associated dark material are present, though mispositioned,
in CEP and OLL
dendrites. Similarly, the disc-shaped accessories normally found at the tips
of the IL1 cilia are present
in the mutant dendrites immediately distal to the neuron/sheath junctions. Striated
rootlets are present in IL1
and OLQ dendrites, some in
their normal position and others in ectopic posterior projections of the dendrite
distal to the neuron/ sheath junctions. The fingers of the AFD
neuron are normal. Abnormal large matrix-filled vesicles accumulate in the amphid
sheath cell.
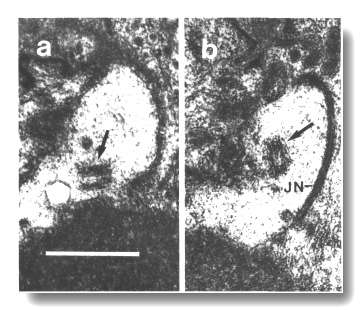 Figure 17. Unmodified centrioles in amphid dendrite of daf-19
(m86) mutant. (a) Section near the termination of a sensory dendrite in
the amphid sheath cell. A centriole with no membrane associations is shown by
an arrow, (b) Section 0.15 micrometer posterior to (a) showing a second centriole
(arrow), oblique to the first centriole, and the neuron/ sheath junction (JN).
Scale bar is 0.5 micrometer.
Figure 17. Unmodified centrioles in amphid dendrite of daf-19
(m86) mutant. (a) Section near the termination of a sensory dendrite in
the amphid sheath cell. A centriole with no membrane associations is shown by
an arrow, (b) Section 0.15 micrometer posterior to (a) showing a second centriole
(arrow), oblique to the first centriole, and the neuron/ sheath junction (JN).
Scale bar is 0.5 micrometer.
che-11 Cilia Contain Abnormal Ground Material
In contrast to the mutants mentioned above, the amphid wing and channel cilia in
che-11 (e1810) are nearly
normal in length and arrangement of microtubules. However, these cilia contain
abnormal dark ground material interspersed among the microtubules of the axoneme (Fig. 18). Some of the cilia are slightly enlarged in diameter and
irregular in contour. The dendrites below the cilia also contain dark ground
material and few, if any, membrane-attached microtubules. The AWC cilia fail to
spread into wing-shaped sheets. Abnormal large matrix-filled vesicles accumulate
in the amphid sheath cell. In one sensillum examined, many of the intermediate
filaments in the sheath scaffold are oriented circumferentially rather than
longitudinally.
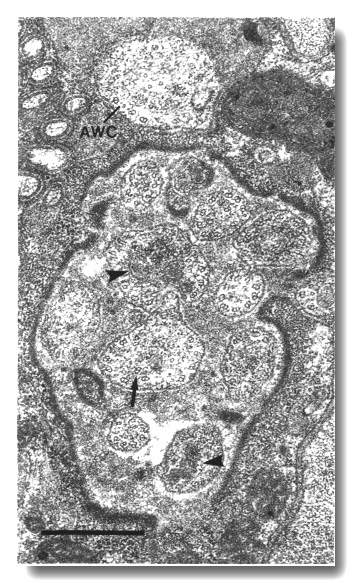 Figure 18. Amphid cilia in che-11 (e1810) mutants.
Section through the anterior sheath channel showing the middle segments of the
channel cilia and the AWC cilium. Many of the cilia are enlarged and irregular
in contour and contain abnormal dark ground material (arrowheads). The doublet
and singlet microtubules of the axoneme are present and nearly normal. An abnormal,
detached doublet (arrow) is visible in one of the channel cilia. The AWC
cilium has failed to spread into wing-shaped sheets. Scale bar is 0.5 micrometer.
Figure 18. Amphid cilia in che-11 (e1810) mutants.
Section through the anterior sheath channel showing the middle segments of the
channel cilia and the AWC cilium. Many of the cilia are enlarged and irregular
in contour and contain abnormal dark ground material (arrowheads). The doublet
and singlet microtubules of the axoneme are present and nearly normal. An abnormal,
detached doublet (arrow) is visible in one of the channel cilia. The AWC
cilium has failed to spread into wing-shaped sheets. Scale bar is 0.5 micrometer.
The CEP cilia
in che-11 (e1810) mutants are reduced in length and largely resemble
the cilia in che-13, osm-1, osm-5, and osm-6. Dark material and
associated microtubules assemble in both rod- and ball-shaped aggregates along
the dendrites and in ectopic posterior projections. The transition zones are
often displaced. Empty tunnels are present in the subcuticle distal to the cuticle-attached
nubbin. In contrast to the other four mutants, the posterior projections are
filled with dark ground material and numerous vesicles.
The IL1,
IL2, OLL, and OLQ
axonemes are nearly normal in length and the transition zones are positioned
correctly in che-11 (e1810). The joined squares in the OLQ
cilia are oriented normally but the flanking dark material is fragmented and
mispositioned. The distal segments of some OLQ
cilia have unattached singlet microtubules in addition to the joined square.
The dark discs of the IL1
cilia and the amorphous dark material in the OLL
cilia were positioned normally. A few membrane-attached singlet microtubules
were found below the IL1
cilia.
che-10 Mutants Lack Amphid Cilia and Striated Rootlets
Most of the amphid wing and channel dendrites in che-10 (e1809) mutants
have no recognizable transition zones or axonemes. These dendrites generally
have enlarged bulb-shaped endings filled with dark ground material (Fig. 19b).
However, usually one or two dendrites per sensillum have well-formed cilia with
normal transition zones and nearly full-length axonemes (Fig. 19a). The wing-shaped
sheets of the AWC cilia are
present. The AFD cilia are
absent or tilted but the fingers are normal. Abnormal large matrix-filled vesicles
accumulate in the sheath cell.
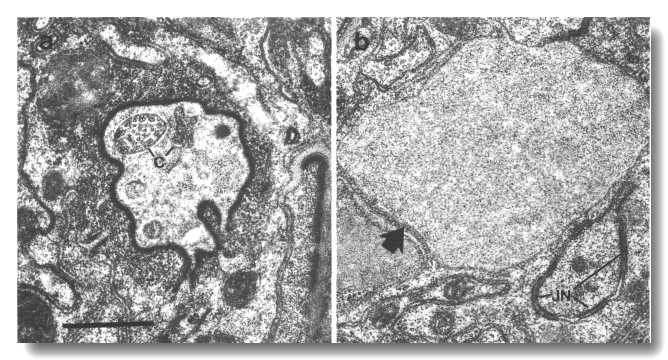 Figure 19. Amphid channel cilia in che-10 (e1809).
(a) Section through the anterior amphid sheath channel. A single cilium (c)
with fairly normal appearance is present in the lumen as are possible remnants
of other cilia, (b) Section 3.5 micrometer posterior to (a). An irregular belt
junction (JN) joins a dendrite to the sheath cell. Another dendrite, sectioned
distal to its junction with the sheath cell, terminates in a large swelling
filled with ground material (arrow). No ciliary structure is evident in either
dendrite. Scale bar is 0.5 micrometer.
Figure 19. Amphid channel cilia in che-10 (e1809).
(a) Section through the anterior amphid sheath channel. A single cilium (c)
with fairly normal appearance is present in the lumen as are possible remnants
of other cilia, (b) Section 3.5 micrometer posterior to (a). An irregular belt
junction (JN) joins a dendrite to the sheath cell. Another dendrite, sectioned
distal to its junction with the sheath cell, terminates in a large swelling
filled with ground material (arrow). No ciliary structure is evident in either
dendrite. Scale bar is 0.5 micrometer.
The striated rootlets normally found at the base of the cilia
in the IL1 (Fig. 20), OLQ,
and BAG neurons are entirely
missing in the mutant che-10 (e1809). The distal specializations of these
cilia, and the other mechanosensory cilia of the head, are normal.
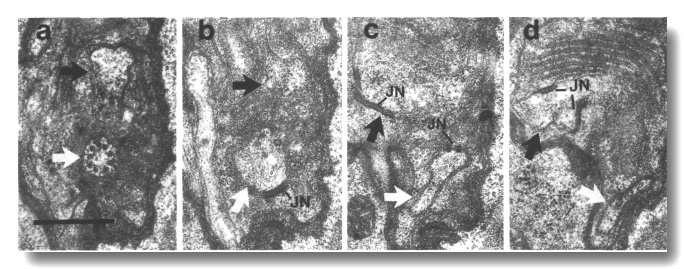 Figure 20. IL1cilium
in che-10 (e1809). (a) Section through the transition zone of an IL1
cilium (white arrow) in che-10 (e1809). No rootlet is seen in the center
of the cilium. The IL2 dendrite
(black arrow) is also visible. (b-d) Sections 0.3, 0.9, and 1.0 micrometer posterior
to (a), respectively, showing that the IL1
dendrite lacks a striated rootlet. Neuron/sheath junctions (JN) are present
on both IL1 and
IL2 dendrites. Scale bar is 0.5 micrometer.
Figure 20. IL1cilium
in che-10 (e1809). (a) Section through the transition zone of an IL1
cilium (white arrow) in che-10 (e1809). No rootlet is seen in the center
of the cilium. The IL2 dendrite
(black arrow) is also visible. (b-d) Sections 0.3, 0.9, and 1.0 micrometer posterior
to (a), respectively, showing that the IL1
dendrite lacks a striated rootlet. Neuron/sheath junctions (JN) are present
on both IL1 and
IL2 dendrites. Scale bar is 0.5 micrometer.
osm-3 Specifically Required for Amphid and Phasmid Cilia
The distal segments of the amphid channel neurons are absent in osm-3 (p802)
mutants (Fig. 21). Both the transition zones and middle segments are normal
in length and contain a full complement of membrane-linked doublet and central
singlet microtubules. The cilia end abruptly, however, in the region where the
B subfibers normally terminate. Thus the distal segments, containing only A
subfibers and central singlets, are entirely truncated and the socket channel
is empty of cilia.
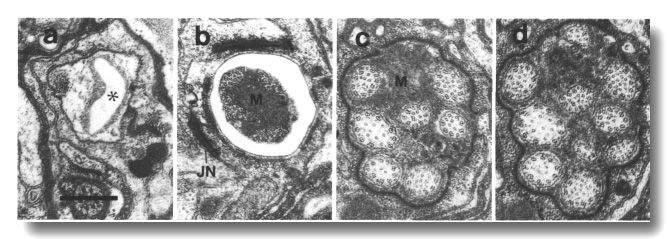 Figure 21. Amphid channel cilia in osm-3 (p802) mutant,
(a) Section through amphid socket cell. The channel (star) is empty of cilia,
(b) Section 2.0 micrometer posterior to (a) at the junction between the sheath
and socket cells (JN). Only four cilia extend this far in the channel. The center
of the channel is occupied by matrix (M). (c, d) Sections 2.7 and 3.0 micrometer
posterior to (a) through the amphid sheath cell. All ten channel cilia are present
in (d).
Figure 21. Amphid channel cilia in osm-3 (p802) mutant,
(a) Section through amphid socket cell. The channel (star) is empty of cilia,
(b) Section 2.0 micrometer posterior to (a) at the junction between the sheath
and socket cells (JN). Only four cilia extend this far in the channel. The center
of the channel is occupied by matrix (M). (c, d) Sections 2.7 and 3.0 micrometer
posterior to (a) through the amphid sheath cell. All ten channel cilia are present
in (d).
Because the channel cilia in osm-3 (p802) have normal middle
segments, they are substantially longer than the che-13, osm-1, osm-5,
and osm-6 cilia. Moreover, the cilia are not displaced forward in the
sheath cell as in the mutants without middle segments. Finally, no ectopically
assembled membrane-linked microtubules are found in osm-3 (p802) dendrites.
The amphid wing cilia are essentially normal in osm-3 (p802).
Similarly, the AFD dendrites,
and the various mechanosensilla, are also normal. The only defect in osm-3
(p802) besides the distal truncation of the amphid channel cilia, is an
accumulation of abnormal, large matrix-filled vesicles in the anterior cytoplasm
of the sheath cell (Fig. 22).
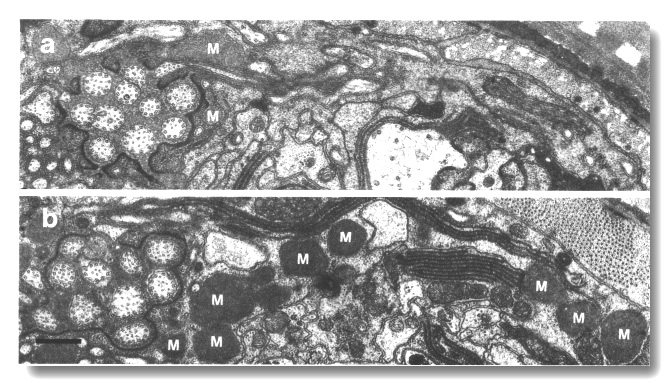 Figure 22. Matrix in amphid sheath cells of wild-type and
osm-3 mutant, (a) Section through the amphid sheath cell in wild-type
showing a few matrix-filled vesicles (M) fusing with the channel lumen. (b)
Comparable section through osm-3 (p802) showing an abnormal accumulation
of large matrix-filled vesicles throughout the sheath cell cytoplasm. Scale
bar is 0.5 micrometer.
Figure 22. Matrix in amphid sheath cells of wild-type and
osm-3 mutant, (a) Section through the amphid sheath cell in wild-type
showing a few matrix-filled vesicles (M) fusing with the channel lumen. (b)
Comparable section through osm-3 (p802) showing an abnormal accumulation
of large matrix-filled vesicles throughout the sheath cell cytoplasm. Scale
bar is 0.5 micrometer.
che-12 Affects the Amphid Sheath Matrix
The matrix vesicles of the amphid sheath cell appear pale or empty in che-12
(e1812). The lumen of the sheath channel and the extracellular space surrounding
the AFD fingers are devoid of matrix. The amphid wing and channel cilia, particularly
near the membrane, are abnormally dark (Fig. 23). The channel cilia are shorter
than normal and only extend partway through the socket channel. Unlike other
mutants with shortened cilia, no large matrix vesicles accumulate in the sheath
cytoplasm.
Irregular vesicles are present between the two layers of the adult cuticle in
che-12 (e1812) (Fig. 23).
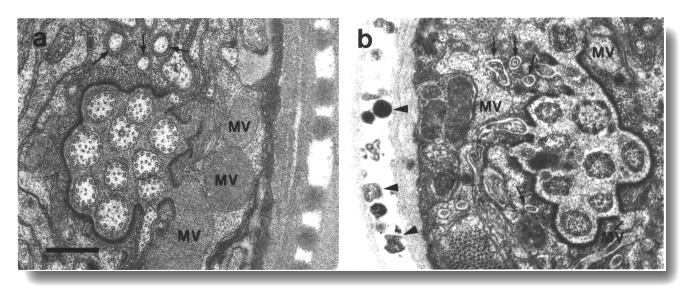 Figure 23. Amphid sheath channel in wild type and che-12
mutant, (a) Wild-type amphid sheath cell showing matrix-filled vesicles (MV)
fusing with channel. The ten cilia in the channel are also surrounded by matrix.
Fingers of the AFD neuron
are shown by arrows, (b) Comparable section from che-12 (e1812) mutant.
The matrix vesicles (MV) appear pale or empty. The channel appears devoid of
matrix and the channel cilia are abnormally dark. The extracellular space between
the sheath cell and the AFD
fingers (arrows) is abnormally pale. Abnormal vesicles (arrowheads) are found
between the layers of the cuticle. Scale bar is 0.5 micrometer.
Figure 23. Amphid sheath channel in wild type and che-12
mutant, (a) Wild-type amphid sheath cell showing matrix-filled vesicles (MV)
fusing with channel. The ten cilia in the channel are also surrounded by matrix.
Fingers of the AFD neuron
are shown by arrows, (b) Comparable section from che-12 (e1812) mutant.
The matrix vesicles (MV) appear pale or empty. The channel appears devoid of
matrix and the channel cilia are abnormally dark. The extracellular space between
the sheath cell and the AFD
fingers (arrows) is abnormally pale. Abnormal vesicles (arrowheads) are found
between the layers of the cuticle. Scale bar is 0.5 micrometer.
che-14 Affects the Joining of the Amphid Channels
The amphid channel is abnormally large in diameter and poorly aligned at the
join between the sheath and socket cells in che-14 (e1960) mutants. The
socket scaffold is disorganized and some of the intermediate filaments are oriented
circumferentially rather than longitudinally. The socket cytoplasm contains
abnormal vesicles and the cuticle lining of the channel is abnormally thin.
The sheath scaffold is apparently stretched thin near the join and the dark
lining of the channel is absent. More posteriorly in the sheath cell, the scaffold
and dark lining appear normal. The belt junction between the sheath and socket
cells is normal.
In some cases, the socket channel fails to connect with the sheath channel and
ends as a blind, cuticle-lined pocket (Fig. 24). When the cilia, which form a
normal fascicle in the sheath, reach an obstructed socket channel, they are
either deflected sideways in the sheath cell or invaginate the socket cell
without obtaining access to the externally open channel (Fig. 25). Matrix
accumulates in the sheath around the distal ends of the deflected fascicles.
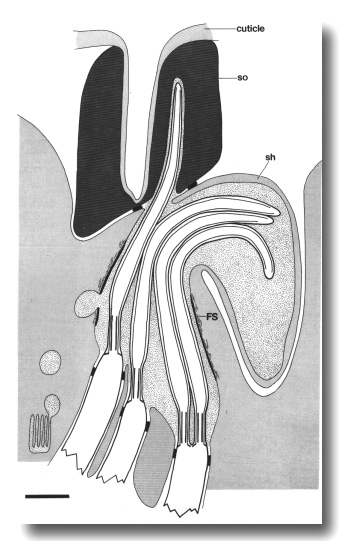 Figure 24. Schematic longitudinal section of an amphid sensillum
in che-14 (e1960). The cilia form a normal fascicle in the sheath cell.
The sheath and socket channels connect aberrantly or, as shown here, fail to
connect. In this case, the cuticle-lined socket channel ends as a blind pocket.
The filamentous scaffold (FS) in both sheath and socket cells is disorganized
and the dark lining that surrounds the anterior sheath channel is missing near
the join of the sheath (sh) and socket (so) cells. Cilia either deflect sideways
in the sheath cell or invaginate the cytoplasm of the socket cell. Scale bar
is 1.0 micrometer.
Figure 24. Schematic longitudinal section of an amphid sensillum
in che-14 (e1960). The cilia form a normal fascicle in the sheath cell.
The sheath and socket channels connect aberrantly or, as shown here, fail to
connect. In this case, the cuticle-lined socket channel ends as a blind pocket.
The filamentous scaffold (FS) in both sheath and socket cells is disorganized
and the dark lining that surrounds the anterior sheath channel is missing near
the join of the sheath (sh) and socket (so) cells. Cilia either deflect sideways
in the sheath cell or invaginate the cytoplasm of the socket cell. Scale bar
is 1.0 micrometer.
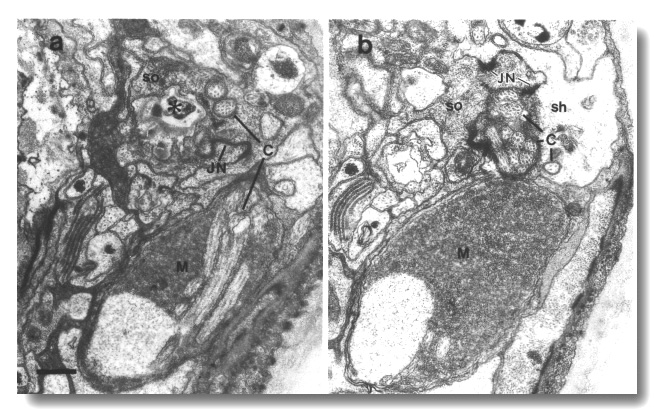 Figure 25. Amphid cilia in che-14 (e1960) mutant,
(a) Section through the amphid socket cell (so). The cuticle-lined channel (star)
ends blindly without connecting to the channel of the sheath cell. The self-junction
(JN) of the socket cell is still formed. The main fascicle of channel cilia
(C) is deflected laterally in the sheath cell and ends blindly in a large deposit
of matrix (M) surrounded by a thin sheet of sheath cell cytoplasm. Two cilia
(C) separate from the main fascicle, exit the sheath cell, and invaginate the
cytoplasm of the socket cell, (b) Section 0.45 micrometer posterior to (a) through
junction (JN) of the sheath (sh) and socket (so) cells. Scale bar is 0.5 micrometer.
Figure 25. Amphid cilia in che-14 (e1960) mutant,
(a) Section through the amphid socket cell (so). The cuticle-lined channel (star)
ends blindly without connecting to the channel of the sheath cell. The self-junction
(JN) of the socket cell is still formed. The main fascicle of channel cilia
(C) is deflected laterally in the sheath cell and ends blindly in a large deposit
of matrix (M) surrounded by a thin sheet of sheath cell cytoplasm. Two cilia
(C) separate from the main fascicle, exit the sheath cell, and invaginate the
cytoplasm of the socket cell, (b) Section 0.45 micrometer posterior to (a) through
junction (JN) of the sheath (sh) and socket (so) cells. Scale bar is 0.5 micrometer.
The cuticle at the tip of the head in che-14 (e1960) is
thin and irregular. The hypodermis, which is pale and somewhat distended, reveals
numerous aggregates of longitudinal intermediate filaments (Fig. 26). Presumably
similar filaments are present in the wild-type hypodermis.
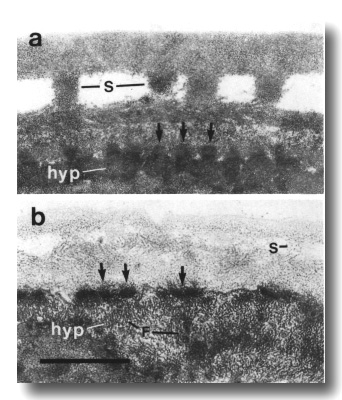 Figure 26. Cuticle and hypodermis in wild type and che-14
mutant, (a) Section 5 micrometer from tip of head in wild-type adult. Struts
(S) join the two layers of the adult cuticle. The hypodermis (hyp) is thin and
dark and is attached to the subcuticle by hemidesmosomes (arrows), (b) Comparable
section through che-14 (e1960) mutant. The cuticle is thin and irregular.
The hypodermis is pale and possibly expanded. Numerous aggregates of intermediate
filaments (F) fill the hypodermal cytoplasm. Scale bar is 0.5 micrometer.
Figure 26. Cuticle and hypodermis in wild type and che-14
mutant, (a) Section 5 micrometer from tip of head in wild-type adult. Struts
(S) join the two layers of the adult cuticle. The hypodermis (hyp) is thin and
dark and is attached to the subcuticle by hemidesmosomes (arrows), (b) Comparable
section through che-14 (e1960) mutant. The cuticle is thin and irregular.
The hypodermis is pale and possibly expanded. Numerous aggregates of intermediate
filaments (F) fill the hypodermal cytoplasm. Scale bar is 0.5 micrometer.
The cuticle-embedded specializations of certain mechanocilia are
abnormal in che-14 (e1960). The discs at the tips of the IL1
cilia are tilted. The nubbins of the CEP
and OLQ dendrites are recessed
in cuticular tunnels (Fig. 27). The joined squares of the OLQ
cilia are sometimes misoriented and, even when the squares are oriented normally,
the dark material that normally flanks the circumferential corners occurs in
abnormally small pieces and is positioned randomly.
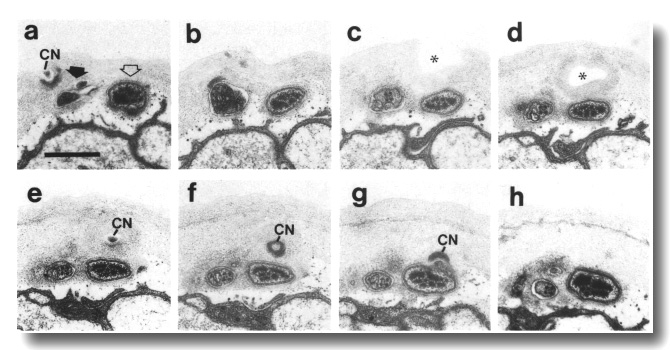 Figure 27. CEP
and OLQ cilia in che-14.
(e1960) mutant, (a-h) Series of sections taken at approximately 0.1-micrometer
intervals from anterior to posterior through the distal segments of the CEP
(open arrow) and the OLQ
(black arrow) cilia. The cuticle-associated nubbin (CN) of the OLQ
cilium completely penetrates the cuticle (a). The joined square of doublet microtubules
is misoriented (c-h) and the cuticle is abnormally thick. The cuticle-associated
nubbin (CN) of the CEP cilium
also penetrates the cuticle (e) and ends at the base of a deep, cuticle-lined
pit (star in c, d). Scale bar is 0.5 micrometer.
Figure 27. CEP
and OLQ cilia in che-14.
(e1960) mutant, (a-h) Series of sections taken at approximately 0.1-micrometer
intervals from anterior to posterior through the distal segments of the CEP
(open arrow) and the OLQ
(black arrow) cilia. The cuticle-associated nubbin (CN) of the OLQ
cilium completely penetrates the cuticle (a). The joined square of doublet microtubules
is misoriented (c-h) and the cuticle is abnormally thick. The cuticle-associated
nubbin (CN) of the CEP cilium
also penetrates the cuticle (e) and ends at the base of a deep, cuticle-lined
pit (star in c, d). Scale bar is 0.5 micrometer.
Like the CEP
neurons, the ADE and PDE
neurons fill with fluorescein in che-14 (e1960) mutants (Table 1). This
suggests that defects in hypodermis and cuticle may extend along the entire
length of the animal.
mec-8 Affects Fasciculation of the Amphid Cilia
In mec-8 (e398), the amphid wing and channel dendrites invaginate the sheath
cell at staggered levels, usually posterior to normal, and their cilia, though
normal in length and ultrastructure, fail to fasciculate (Fig. 28). Individual
cilia and partial fascicles course separately through the sheath cell and
accrete matrix, dark lining, and scaffold material (Fig. 29). Some cilia turn
laterally or even posteriorly and most end blindly within the sheath cell. The
belt junctions connecting the amphid socket and sheath cells are mispositioned
and the cuticle-lined channel of the socket cell sometimes ends in a blind
pocket without opening onto a channel in the sheath cell. Channel cilia reaching
the socket cell may invaginate it without obtaining access to the externally
open channel. Abnormal large matrix-filled vesicles accumulate in the sheath
cells.
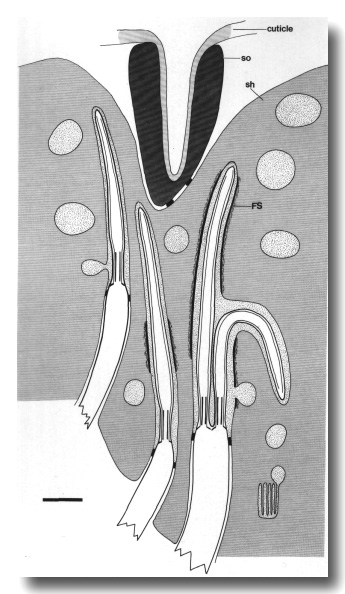 Figure 28.Schematic longitudinal section through the amphid
sensillum in mec-8 (e398). The wing and channel cilia fail to form a
single fascicle within the sheath (sh) cell. Instead, they course separately
or in small fascicles, accreting matrix, and, sometimes, the dark lining and
filamentous scaffold (FS) material that surround the anterior sheath channel
in wild-type. The cuticle-lined channel of the socket (so) cell may end in a
blind pocket rather than connecting to any of the fascicles in the sheath cell.
Scale bar is 1.0 micrometer.
Figure 28.Schematic longitudinal section through the amphid
sensillum in mec-8 (e398). The wing and channel cilia fail to form a
single fascicle within the sheath (sh) cell. Instead, they course separately
or in small fascicles, accreting matrix, and, sometimes, the dark lining and
filamentous scaffold (FS) material that surround the anterior sheath channel
in wild-type. The cuticle-lined channel of the socket (so) cell may end in a
blind pocket rather than connecting to any of the fascicles in the sheath cell.
Scale bar is 1.0 micrometer.
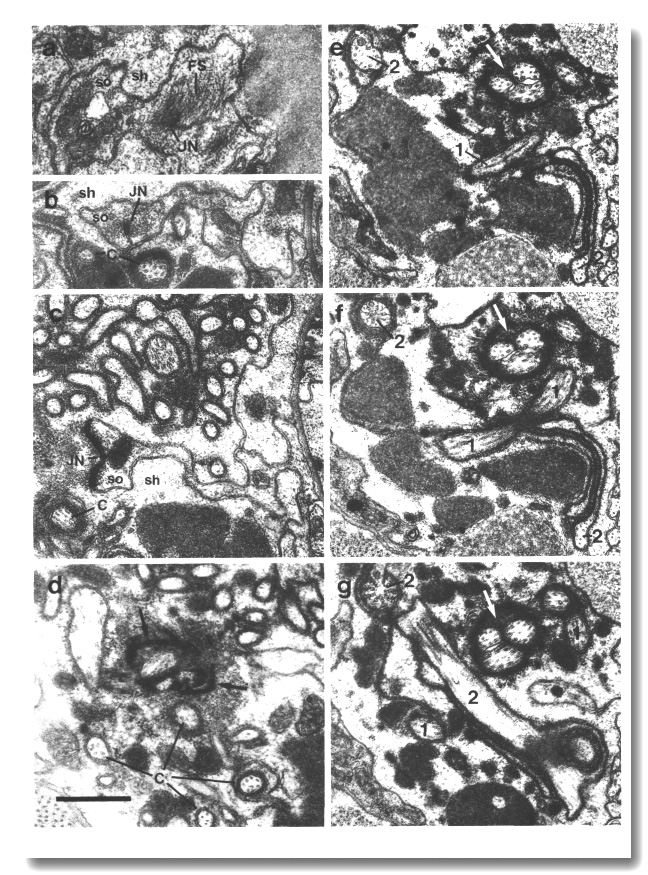 Figure 29. Amphid sensillum in mec-8 (e398). (a, b)
Sections through the socket cell (so) showing disarrayed intermediate filaments
(FS) of the scaffold associated with a self-junction (JN). The cuticle-lined
channel has failed to extend this far posteriorly. A few isolated cilia (C)
are visible in the sheath cell (sh). (c) Section 1.3 micrometer posterior to
(b) showing sheath/socket junction (JN). The cilium and fingers of the AFD
dendrite are visible as is an isolated channel cilium (C). (d) Section 1.6 micrometer
posterior to (b) showing four isolated channel cilia (C) and the distal end
of a fascicle of three cilia. The fascicle is surrounded by the matrix material,
dark lining (black arrows), and filamentous scaffold that surround the channel
cilia in wild-type, (e-g) Sections 6.5, 6.7, and 6.9 micrometer posterior to
(b). Three cilia form a fascicle (white arrow). The cilium of another neuron
(1) makes a complete U-turn and extends posteriorly into the sheath cell. The
paired cilia of another neuron (2), probably
AWB, are orthogonal, rather than parallel, at their bases. Scale bar is
0.5 micrometer.
Figure 29. Amphid sensillum in mec-8 (e398). (a, b)
Sections through the socket cell (so) showing disarrayed intermediate filaments
(FS) of the scaffold associated with a self-junction (JN). The cuticle-lined
channel has failed to extend this far posteriorly. A few isolated cilia (C)
are visible in the sheath cell (sh). (c) Section 1.3 micrometer posterior to
(b) showing sheath/socket junction (JN). The cilium and fingers of the AFD
dendrite are visible as is an isolated channel cilium (C). (d) Section 1.6 micrometer
posterior to (b) showing four isolated channel cilia (C) and the distal end
of a fascicle of three cilia. The fascicle is surrounded by the matrix material,
dark lining (black arrows), and filamentous scaffold that surround the channel
cilia in wild-type, (e-g) Sections 6.5, 6.7, and 6.9 micrometer posterior to
(b). Three cilia form a fascicle (white arrow). The cilium of another neuron
(1) makes a complete U-turn and extends posteriorly into the sheath cell. The
paired cilia of another neuron (2), probably
AWB, are orthogonal, rather than parallel, at their bases. Scale bar is
0.5 micrometer.
The various mechanosensilla of the head are normal in mec-8 (e398).
cat-6 Affects the CEP Specializations
The transition zones and middle segments of the CEP
cilia in cat-6 (e1861) are positioned slightly anterior of normal but
are normal in length. The distal specializations, supernumerary microtubules
and associated dark material, form normal rod-shaped aggregates. These rods,
however, are not confined to the distal segments but assemble along the entire
cilia as well as ectopically, proximal to the cilia (Figs. 30, 31d, e). The
cuticle-attached nubbins may also contain rods separated from the ciliary shaft.
Such nubbins are enlarged and often extend completely through the cuticle (Figs.
30, 31a-c).
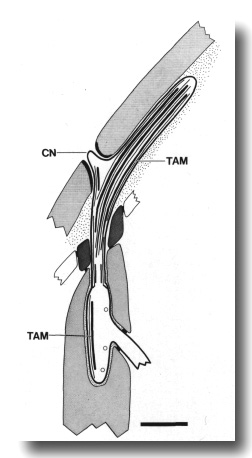 Figure 30. Schematic longitudinal section through CEP
cilium in cat-6 (e1861). Rod-shaped aggregates of tubule-associated material
(TAM) and supernumerary microtubules assemble along the entire cilium and below
it. They also extend into the cuticle-attached nubbin (CN) which is larger than
normal and often penetrates the cuticle. Scale bar is 1.0 micrometer.
Figure 30. Schematic longitudinal section through CEP
cilium in cat-6 (e1861). Rod-shaped aggregates of tubule-associated material
(TAM) and supernumerary microtubules assemble along the entire cilium and below
it. They also extend into the cuticle-attached nubbin (CN) which is larger than
normal and often penetrates the cuticle. Scale bar is 1.0 micrometer.
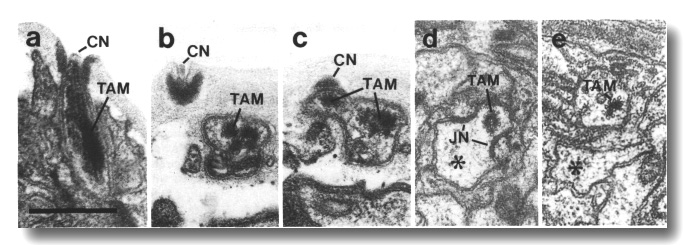 Figure 31. CEP
cilia in cat-6 (e1861) mutants, (a) Longitudinal section through a newly
molted L4 hermaphrodite showing the cuticle-associated nubbin (CN) of a CEP
cilium as it penetrates the cuticle. Unlike wild-type, microtubules and dark
tubule-associated material (TAM) extend into the nubbin, (b) Transverse section
through a CEP cilium of an
adult cat-6 mutant showing the cuticle-associated nubbin (CN) as it penetrates
the cuticle, (c) Section 0.2 micrometer posterior to (b) showing that the tubules
and tubule-associated material (TAM) partition into the cuticle-associated nubbin
(CN) and the main shaft, (d) Section 3.8 micrometer posterior to (b) at the
level of the neuron/sheath junction (JN). A cluster of microtubules and dark
tubule-associated material (TAM) remain within the sheath cell as the main dendrite
(star) exits, (e) Section 4.2 micrometer posterior to (b). Microtubules and
tubule-associated material (TAM) remain in a posterior projection within the
sheath cell. The main dendrite (star) is entirely outside the sheath cell and
devoid of ciliary structures. Scale bar is 0.5 micrometer.
Figure 31. CEP
cilia in cat-6 (e1861) mutants, (a) Longitudinal section through a newly
molted L4 hermaphrodite showing the cuticle-associated nubbin (CN) of a CEP
cilium as it penetrates the cuticle. Unlike wild-type, microtubules and dark
tubule-associated material (TAM) extend into the nubbin, (b) Transverse section
through a CEP cilium of an
adult cat-6 mutant showing the cuticle-associated nubbin (CN) as it penetrates
the cuticle, (c) Section 0.2 micrometer posterior to (b) showing that the tubules
and tubule-associated material (TAM) partition into the cuticle-associated nubbin
(CN) and the main shaft, (d) Section 3.8 micrometer posterior to (b) at the
level of the neuron/sheath junction (JN). A cluster of microtubules and dark
tubule-associated material (TAM) remain within the sheath cell as the main dendrite
(star) exits, (e) Section 4.2 micrometer posterior to (b). Microtubules and
tubule-associated material (TAM) remain in a posterior projection within the
sheath cell. The main dendrite (star) is entirely outside the sheath cell and
devoid of ciliary structures. Scale bar is 0.5 micrometer.
The OLQ cilia
in cat-6 (e1861) may have a reduced amount of dark material flanking
the circumferential corners of the square of doublet microtubules. The other
classes of mechanocilia, including
IL1 and OLL, and the
amphid sensilla appear normal.
ttx-1 Thermosensory Mutants Lack the AFD Fingers
The fingers of the AFD neurons
in the cryophilic mutant ttx-1 (p767; formerly EH67, Hedgecock
and Russell, 1975) are entirely missing. Instead, a fingerless sack of membrane
protrudes from the dendrites just below the cilia (Fig. 32). The cilia are about
4 micrometer long, three times their normal length, and are tilted ventrally
at their bases, away from the anteriorly projected sack (Fig. 33). The amphid
wing and channel cilia and the various mechanosensilla of the head are normal.
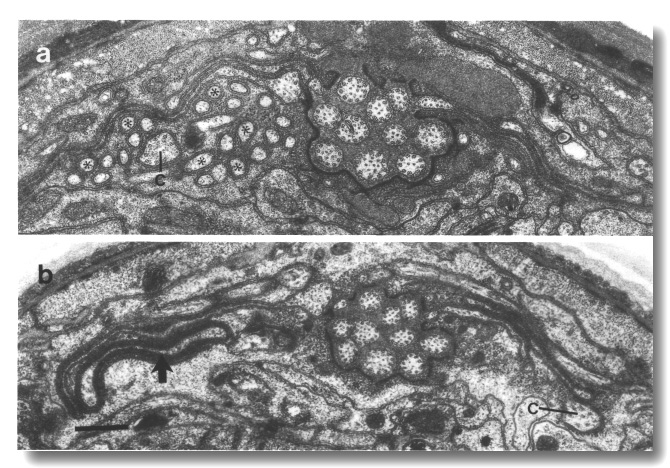 Figure 32. AFD
cilia in wild type and ttx-1 mutant, (a) Section through wild type amphid sheath
cell showing the AFD cilium
(C) and about 25 fingers (stars), (b) Comparable section through ttx-1 (e767)
amphid sheath cell. Distal to the neuron sheath junction, the AFD
dendrite has bifurcated into a fingerless sack (black arrow) and a cilium (C).
The cilium is longer than normal and tilted ventrally. The sack is surrounded
by lamellae of the sheath cell. The channel cilia are completely normal. Scale
bar is 0.5 micrometer.
Figure 32. AFD
cilia in wild type and ttx-1 mutant, (a) Section through wild type amphid sheath
cell showing the AFD cilium
(C) and about 25 fingers (stars), (b) Comparable section through ttx-1 (e767)
amphid sheath cell. Distal to the neuron sheath junction, the AFD
dendrite has bifurcated into a fingerless sack (black arrow) and a cilium (C).
The cilium is longer than normal and tilted ventrally. The sack is surrounded
by lamellae of the sheath cell. The channel cilia are completely normal. Scale
bar is 0.5 micrometer.
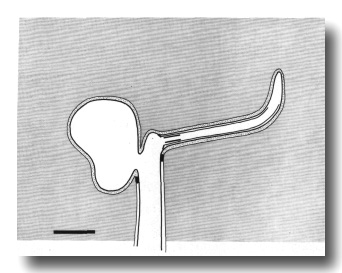 Figure 33. Schematic longitudinal section of AFD
dendrite in ttx-1 (p767). The cilium is tilted ventrally and is longer
than normal. Below the cilium, dendritic membrane protrudes in a fingerless
sack. Scale bar is 0.5 micrometer.
Figure 33. Schematic longitudinal section of AFD
dendrite in ttx-1 (p767). The cilium is tilted ventrally and is longer
than normal. Below the cilium, dendritic membrane protrudes in a fingerless
sack. Scale bar is 0.5 micrometer.
Discussion
Development of the Sensilla
The assembly of an individual sensillum requires interactions between at least
four cell types. One or more neurons invaginate and form junctions with the
sheath cell, the sheath cell forms junctions with the socket cell, and the
socket cell forms junctions with adjacent epidermal cells. Presumably a specific
cell-cell adhesion is required before any permanent junction can form. In the
embryo, many sensilla are assembled concurrently in a small region of the head.
Thus each cell must adhere to its correct partners despite possible competition
from nearby cells of similar type. This specificity of attachment is not
absolute as hybrid sensilla can form when normal partners are removed (Sulston
et al., 1983).
Invagination may be an early step in neuron/sheath interaction.
In daf-19 mutants, where cilia are apparently not formed, the sensory
dendrites still invaginate their sheath cells and form normal belt junctions.
The amphid dendrites show specific neuron/neuron interactions in addition to the
neuron/sheath interactions observed in all sensilla. The 12 dendrites normally
invaginate the sheath cell roughly in register. The channel cilia form a tight
fascicle in the sheath cell which extends into the socket channel. Curiously,
the arrangement of cilia within this fascicle is invariant in wild-type animals
(Ward et al, 1975; Ware et al, 1975). It is unknown whether the ciliary pattern
is inherited from the more complex pattern of the papillary nerves.
In mec-8 mutants, the amphid dendrites invaginate the sheath
cell at irregular levels and their cilia do not fasciculate fully. A similar,
if milder, defect in amphid fasciculation has been observed in mec-1
mutants (Lewis and Hodgkin, 1977; Chalfie and Sulston, 1981). Conceivably the
mec-1 and mec-8 genes specify adhesive molecules that determine
pairwise affinities of the amphid dendrites or their cilia. In addition, the
mec-1 and mec-8 mutations disrupt the function of certain nonciliated
mechanosensory neurons (Chalfie and Sulston, 1981). The mec-1 mutations
were shown to prevent the normal attachment of these neurons to the hypodermis.
The lining and scaffold of the amphid sheath channel assemble
around the fascicle of cilia. The sheath channel forms correctly in mutants
with truncated or missing cilia suggesting that the dendrites, and not exclusively
their cilia, can induce these structures. Small fascicles or isolated cilia
in mec-8 mutants form separate channels that can accrete a scaffold and
dark lining resembling the normal sheath channel.
The sheath matrix material appears to be synthesized at the lamellae,
transported forward in membrane bound vesicles, and secreted from these vesicles
into the sheath channel near the base of the cilia (Wright, 1980). The cilia
themselves appear to induce the deposition of the matrix material. In mec-8
mutants with displaced cilia, the matrix material still deposits along them.
It is also deposited around the ectopic cilia-like projections found in che-13,
osm-1, and osm-5 dendrites.
In mutants with short or absent cilia, matrix material accumulates
in large vesicles in the anterior sheath cytoplasm. Abnormal accumulations of
large matrix vesicles have also been reported in the amphid sheath cells of
che-2, che-3, and daf-6 mutants (Lewis and Hodgkin, 1977; Albert
et al, 1981). It may be that matrix material is normally discharged from the
cilia through the amphid openings. This would explain why it accumulates in
the che-14, daf-6, and mec-8 mutants that have apparently normal
cilia but obstructed channels.
The che-12 mutation appears to disrupt the synthesis or
secretion of matrix by the sheath cells. Interestingly, empty vesicles still
form at the lamellae, transport forward, and fuse with the channel lumen. Presumably
the abnormal darkening of the channel cilia in the che-12 mutants is
a degenerative change resulting from the loss of matrix normally surrounding
the cilia. These mutants also have a defect in cuticle secretion by the epidermis.
The socket channel has a rather different origin than the sheath
channel (Wright, 1980). The socket cells can wrap around and form junctions
with themselves, thus creating a channel, even when there are no cilia to envelop.
The scaffold that assembles around the channel cilia in the sheath cell may
be important in joining the sheath and socket channels. In the absence of a
well defined sheath channel, the socket channel sometimes ends in a blind pocket
in mec-8 mutants. The sheath channel appears nearly normal in che-14
mutants but the join with the socket channel is defective. Conceivably the primary
defect is in the sheath or socket scaffolds. The published description of daf-6
(e1377) mutants suggests they, too, may be defective in the joining of the
sheath and socket channels (Albert et al, 1981). Consistent with this idea,
Herman (1984) has shown that the genetic focus of the daf-6 phenotype
is probably the sheath or the socket cell, or possibly both, but not the neurons
(Table 2).
Assembly of Sensory Cilia
All classes of sensory cilia are absent in daf-19 mutants. Vestigial
centrioles, without membrane attachments, were found in a few dendrites. No
membrane-linked microtubules assemble ectopically in these mutants, suggesting
(see below) that the wild type daf-19 product directly affects the peripheral
doublets or their Y links. A mutation disrupting doublet-microtubules has been
described in Chlamydomonas (Goodenough and St. Clair, 1975).
The che-13, osm-1, osm-5, and osm-6 mutations shorten the
axonemes of all classes of cilia. Microtubules, attached to the membrane by
Y links, assemble ectopically in these mutants. The number and lengths of these
ectopic microtubules vary by neuron type and roughly parallel the normal lengths
of cilia in these cells. We suggest these ectopic microtubules are misassembled
components of the axoneme. Thus the peripheral doublets and Y links can apparently
self-assemble and the wild-type products of these four genes are needed to ensure
they assemble only on the ciliary template. Interestingly, the transition zones
are fairly normal in these mutants. Also, the OLQ
axonemes are probably less affected than other classes. It may be that additional
structures, such as the apical ring in the transition zone and the filled microtubules
or crossbridges in the OLQ
axonemes, increase the stability of these segments even in the absence of normal
che-13, osm-1, osm-5, and osm-6 products. Consistent with the
idea that these genes encode ciliary components, Herman (1984) has shown that
the wild-type osm-1 gene must be expressed in the neurons themselves
for normal cilia as judged by FITC uptake.
All classes of sensory cilia in che-2 and che-3
mutants have been previously shown to have normal transition zones and truncated
axonemes (Lewis and Hodgkin, 1977; Albert et al, 1981). It was reported that
microtubules assemble ectopically below the transition zones in both of these
mutants. Whereas the truncated amphid cilia in che-2, che-13, osm-1, osm-5,
and osm-6 mutants are normal in diameter, the amphid cilia in che-3
mutants have enlarged, bulb-shaped endings filled with dark ground material
(Lewis and Hodgkin, 1977).
The amphid channel cilia of che-11 and daf-10 mutants
are irregular in contour, variably enlarged in diameter, and may contain dark
ground material in the center of the axoneme (Albert et al, 1981). The che-11
channel cilia are nearly normal in length whereas the daf-10 cilia are
described as foreshortened (Albert et al, 1981). Both mutants also affect the
CEP cilia and, at least for
che-11, probably other cilia. Perhaps related, dark ground material has
been observed in the center of the axonemes in the bronchial epithelium of a
human subject with immotile cilia (Afzelius, 1976).
The amphid cilia are usually absent in che-10 mutants.
Instead, the dendrites have large, bulb-shaped endings filled with dark ground
material. Occasional dendrites have well-formed cilia, suggesting the amphid
defect is degenerative rather than developmental. The mechano-cilia appear normal
but lack striated rootlets. It may be interesting to examine the amphid dendrites
in embryos or L1 larvae of this strain.
The osm-3 mutation specifically eliminates the distal segments
of the amphid channel cilia, leaving the middle segment and the transition zone
unaffected. The distal segment differs from the middle segment in that the B
subfibers of the peripheral doublets, and the membrane-links, are absent. The
osm-3 product may be a protein specific to the distal segments of these
cilia. Alternatively, it may affect the entire cilium, perhaps being associated
with the A subfibers, but the distal segment is most vulnerable to its absence.
Dissociation of the IL2 Cilia
In wild-type adults, the IL2
neurons, and possibly some mechanosensory neurons, have incomplete cilia comprising
fewer than nine doublets (Ward et al, 1975; Ware et al., 1975). Interestingly,
in the che-13, osm-1, osm-5, and osm-6 mutants with truncated
cilia, the transition zones of the IL2
cilia and the various mechanocilia are actually longer and better formed, in
the sense of showing nine Y-linked doublet microtubules drawn together in a
ring, than in wild type. We speculate that when they form all classes of cilia
have complete transition zones, but certain classes, particularly the IL2
cilia, later dissociate or rearrange, leaving fewer microtubules and no recognizable
nine-fold organization. Rearrangement might be expected if, for example, the
neurons undersynthesize ciliary proteins during dendrite growth. Mutants which
destabilize the axoneme might actually leave more material available for maintaining
the transition zone than in the wild type. It may be interesting to examine
the IL2 cilia in embryos
or L1 larvae.
Striated Rootlets
Striated rootlets are frequently associated with the basal bodies of both sensory
and motile cilia but their function is unknown. Salisbury and Floyd (1978) have
shown that the rootlets of certain flagellate alga are contractile and that
contraction is induced by calcium. A calcium-binding phosphoprotein of 20,000
MW is the principal component of the contractile rootlets from Tetraselmis
striata (Salisbury et al, 1984). Striated rootlets have also been purified
from several other sources (see Salisbury et al, 1984). In each case, only one
or two proteins account for most of the protein in the purified rootlets. However,
the molecular weights of these proteins vary widely and it remains to be seen
how they are related.
The daf-19 mutation eliminates cilium formation, but striated
rootlets still assemble in the appropriate dendrites. Interestingly, certain
sensory neurons in C. elegans males contain striated rootlets but no
associated cilia (Sulston et al., 1980). The rootlets are attached to dark plates,
resembling hemidesmosomes, at the dendritic tips. These observations imply that
rootlet and cilium formation can occur independently and an unknown mechanism
ensures that the distal end of the rootlet attaches to the base of the cilium.
A similar conclusion has been reached using basal body defective and rootlet
defective mutants of Chlamydomonas (Goodenough and St. Clair, 1975; Wright
et al., 1983). Interestingly, the ectopic membrane-linked microtubules in the
che-13, osm-1, osm-5, and osm-6 mutants can recruit small rootlets.
Presumably, the same affinity exists between the rootlets and microtubules of
normal cilia.
The che-10 mutation eliminates striated rootlets from the mechanosensory
cilia of the head. The wild type che-10 product may be a rootlet component.
The amphid cilia, which lack striated rootlets in the wild-type, are badly degenerated
in this strain. A possible explanation, that the strain harbors two mutations,
one responsible for the rootlet defect and one for the amphid defect, can be
resolved by isolating a second, independent che-10 mutant and examining
its rootlets.
Mechanosensory Specializations and Modalities
The distal tips of the IL1
cilia each contain a dark membrane-attached disc. These discs are positioned
in the cuticle at the base of the papillae in such a way as to be compressed
by head-on contacts of the animal. The mutants with truncated or missing
IL1 cilia show that the discs can assemble normally in the absence of cilia
but they require the cilia to position them at the tip of the dendrite.
The supernumerary microtubules and associated dark material of the CEP
cilia closely resemble the tubular bodies found in proved mechanocilia of insects
(Thurm et al, 1983). The dark material, itself amorphous, appears to be molded
into rods by the associated microtubules. A similar dark material is found at
the tips of the OLL cilia
where it forms a ball. The difference in shape may reflect the comparative paucity
of microtubules in the OLL
cilia. In mutants with truncated or missing cilia, the supernumerary microtubules
and dark tubule-associated material in the CEP neurons assemble ectopically
along the dendrite in both rod- and ball-shaped aggregates. This shows that
these specializations can self-assemble but the cilia are needed to position
them at the distal tip of the dendrite. In the cat-6 mutants, both the CEP
cilia and their specializations appear normal but the association between them
is specifically disrupted.
Amorphous dark material is also present in the OLQ
cilia as small lumps that flank the circumferential corners of the joined square
of microtubules. These lumps may also be connected to the membrane. In the wild-type,
the corners of the square always point radially and circumferentially. There
is no obvious structure joining the OLQ
axoneme to the support cells or the cuticle that might provide orientation.
A simple suggestion is that the square and associated dark material are aligned
passively by repeated deformation of the cuticle as might occur during stimulation.
Interestingly, the OLQ squares
are sometimes misoriented and the dark lumps are fragmented and mispositioned
in che-14 mutants which have abnormally thick subcuticle adjacent to
the cilia. Perhaps relevant, the cilia of the respiratory epithelia, which normally
are oriented to beat in a common direction, are randomly oriented, as judged
by their basal feet, in subjects with immotile cilia (Afzelius, 1981). Here,
it is thought that cilia form in random orientations and become oriented through
a mechanism involving active beating.
As the CEP,
OLL, and OLQ
cilia are situated somewhat posterior to the IL1
cilia, they probably detect radial, rather than axial, displacements. The geometry
of the OLQ cilia suggests
they have substantial directional discrimination. The adjacent CEP
cilia may be lower threshold, isotropic detectors.
The dark, cuticle-embedded nubbins of the CEP,
OLL, and OLQ
dendrites presumably provide mechanical anchorage of the dendrite to the cuticle.
They are not a ciliary specialization as such since they persist in mutants
with truncated cilia and, at least for the CEP
dendrites, in the daf-19 mutants without cilia. In the cat-6 mutants
with enlarged CEP nubbins
or the che-14 mutants with abnormally thin cuticle, the nubbins can completely
penetrate the cuticle and expose the dendrite to the medium.
A similar cuticle-embedded nubbin occurs in males at the distal
tips of the CEM cilia. Here, it penetrates the cuticle and is believed to provide
access of the CEM dendrite to the chemical environment. As there is no cuticular
opening in the cephalic sensilla of hermaphrodites which lack the CEM neurons,
the openings in males must be created by the CEM dendrites and not the cephalic
socket cells. In contrast, the raised cuticle and pore of the inner labial sensilla
appear to be formed by the innerlabial socket cell. They persist in mutants
with truncated or missing IL1
and IL2 cilia.
Chemosensory Behaviors
C. elegans has at least five distinct chemosensory behaviors. First,
it is attracted or repelled by a variety of small molecules at low concentrations
(10 -3 M or below) (Ward, 1973; Dusenbery, 1974, 1975, 1976a, 1980a,c).
Second, it is repelled by very high concentrations of various chemically unrelated
solutes, including NaCl and fructose (Ward, 1973; Culotti and Russell, 1978).
Third, when starved under crowded conditions, young larvae may differentiate
into dauer larvae, a non-feeding, arrested stage, adapted for long-term survival
and dispersal (Cassada and Russell, 1975). Crowding is sensed by the accumulation
of a fatty-acid-like pheromone made constitutively by all animals (Golden and
Riddle, 1982, 1984a,b). Fourth, chemical cues influence egg laying (Horvitz
et al, 1982; Golden and Riddle, 1982; Trent et al, 1983). Fifth, males are attracted
to hermaphrodites by an unknown attractant (H. Horvitz and J. Sulston, personal
communication). The cephalic companions (CEM), a class of chemosensory neurons
found only in males, may be detectors for an hermaphrodite pheromone.
The amphid and phasmid sensilla have long been suspected of mediating
many of these chemosensory behaviors (Wright, 1980). Each class of neurons in
these sensilla has distinct synaptic outputs, suggesting their cilia may detect
different chemicals (Hall and Russell, 1986; White et al, 1986). The clearest
evidence that the ten classes of channel cilia are required for chemotaxis,
osmotic avoidance, and dauer larvae formation comes from the mutants osm-3,
(p802), and daf-6 (el377). The ultrastructural defects in the heads of
these mutants are confined to the amphid channel cilia and amphid sheath cell,
respectively (Albert et al, 1981). These mutants fail to form dauer larva in
response to pheromone, to avoid concentrated NaCl or fructose, or to chemotax
toward dilute NaCl (Culotti and Russell, 1978; Albert et al, 1981).
Interestingly, osm-3 (p802) mutants are still responsive
to some chemicals including pyridine, C02 and H+ (Dusenbery, 1980b).
This may reflect some residual responsiveness of the shortened channel cilia.
Alternatively, the amphid wing neurons or the
IL2 neurons may be the principal detectors for these chemical species. Significantly,
the osm-1 (p808) mutation, which affects the assembly of all classes
of cilia, abolishes even these responses (Dusenbery, 1980b). Hence, all known
chemical attractants, repellants, and pheromones are apparently sensed through
ciliated receptors in C. elegans.
Competing levels of food and crowding pheromome are believed to
regulate both entry into and exit from the dauer larval stage. Mutants with
abnormal amphid cilia are generally incapable of forming dauer larva in response
to crowding and starvation (Tables 1 and 2) or direct application of pheromome
(Golden and Riddle, 1984a). Two of these mutants, daf-6 and daf-10,
have been induced to form dauer larva by introducing second mutations which
favor dauer formation (Albert et al, 1981; Riddle et al, 1981). In these genetic
backgrounds, the daf-6 and daf-10 mutations inhibit exit from
the dauer stage perhaps by preventing detection of a food signal. Paradoxically,
the daf-19 mutants with no sensory cilia form dauer larva constitutively
in the absence of crowding or starvation (D. Riddle, personal communication).
This suggests that mutations affecting the sensory cilia can shift decisions
to enter or exit the dauer stage in either direction
Horvitz et al. (1982) have reported that che-3, daf-10,
and osm-3 mutants lack biochemically detectable levels of octopamine,
a presumptive neurotransmitter found in the wild-type. The common defect of
these three mutants is a disruption of the amphid and phasmid cilia. This suggests
that these neurons either make octopamine or regulate the neurons which do.
Mating Behavior
Mating by C. elegans males is a complex behavior involving ten classes
of male-specific sensory neurons (Ward et al, 1975; Sulston et al, 1980; Hodgkin,
1983). Ten genes that affect mechanosensory receptors in the head, che-2,
che-3, che-10, che-11, che-13, daf-10, daf-19, osm-1, osm-5, and osm-6,
are also required for mating (Table 1). These mutations likely prevent mating
by disrupting male-specific sensilla in the tail. Most of these mutants show
occasional fluorescein uptake into ray neurons, indicating that the ray sensilla
are abnormal. The mating defect in che-10 (e1809) may be the consequence
of missing striated rootlets normally found in dendrites of the ray, hook, and
postcloacal sensilla (Sulston et al, 1980).
As expected, the various neurons of the amphid and phasmid sensilla
are probably not important for male mating behavior as che-12, daf-6, osm-3,
and ttx-1 mutants all mate efficiently (Table 1). Similarly, the efficient
mating of cat-6 males implies that the ADE,
CEP, and PDE
neurons are not involved.
Possible Thermosensory Role of Amphid Finger Neuron (AFD)
The AFD dendrites are unique
among sensory receptors in C. elegans in having numerous fingers that
invaginate the surrounding sheath cell. These fingers, which are topologically
proximal to the AFD cilia,
do not depend on the cilia for formation since mutants with reduced axonemes
(che-13, osm-1, osm-5, and osm-6) or no cilia (daf-19) have normal
fingers.
R. Ware has suggested, based on his unpublished observations on
ttx-1 (p767) mutants, that the AFD
neurons may be thermosensory. As confirmed here, the AFD
fingers are entirely missing and the AFD
cilia are longer than normal in ttx-1 mutants. The other sensory receptors
in the head appear ultrastructurally normal.
When placed in a thermal gradient, wild-type animals move toward
the temperature at which they were previously raised (Hedgecock and Russell,
1975). The ttx-1 thermotaxis mutants seek the cold regardless of their
thermal history. These mutants are also hyper-responsive to dauer-inducing pheromone
(Golden and Riddle, 1984a,b). Elevated temperatures are known to lower the pheromone
threshold for dauer-larva formation in wild-type animals. This suggests that
both the cryophilia and heightened pheromone sensitivity of the ttx-1
mutants may reflect a common sensory defect in which the animal perceives a
higher temperature than actual. Other sensory behaviors, including chemotaxis
and mating, are normal in this strain (Hedgecock and Russell, 1975; Dusenbery
and Barr, 1980).
A reduction in the number of fingers on the
AFD dendrites has also been reported for the che-1 mutants, e1034
and a74 (formerly DD74) (Lewis and Hodgkin, 1977; R. Ware, D. Clark,
M. Salzay, and R. Russell, personal communication). No thermotaxis defects were
detected in population assays of che-1 mutants (Hedgecock and Russell,
1975). However, the finger abnormality in che-1 mutants is variable and
comparatively mild. About half of the che-1 (e1034) animals examined
by Lewis and Hodgkin (1977), for example, had normal or nearly normal AFD
dendrites.
It may be possible to confirm a role for the AFD
neurons in thermal behavior by killing these cells with a laser microbeam (Sulston
and White, 1980) and testing the animals in individual thermotaxis assays (Hedgecock
and Russell, 1975).
Photosensory Behavior
Burr (1985) has reported that C. elegans responds to light by reversing,
and consequently changing the direction of movement, more frequently than in
the dark. This is a nonoriented response but, in principle, could be used to
keep animals away from lighted areas (Fraenkel and Gunn, 1961). The light appears
to act directly and not by radiant heating.
In nematodes with true phototaxis, the ocelli comprise a pair
of amphid dendrites plus nearby pigment spots in the pharynx which provide shadowing
(see Burr, 1985). Although C. elegans lacks obvious photopigments or
shadowing pigments, the AWC
neurons are plausible candidates for photoreceptors as their cilia have extremely
large membrane areas. Tests of mutants such as daf-19 may help ascertain
whether the photoresponse in C. elegans is mediated by ciliated sensory
neurons.
Evolution of Sensory Cilia
Motile cilia, found in unicellular eukaryotes, lower plants, and animals,
are believed to be ancient organelles. The sensory cilia of animals probably
arose by later modification of motile cilia. In nematodes, the motile functions
of cilia have apparently been lost. Their spermatozoa are nonflagellated and
move by extending contractile pseudopodia (Ward et al, 1982), and there are
no ciliated epithelia. In contrast, the sensory functions of cilia are highly
elaborated. Wright (1983) has suggested, that since there is no selective pressure
to maintain ciliary structures used strictly for motility, nematode cilia may
be simpler than in other animals. For example, the dynein and nexin arms, radial
spokes, and central pair of singlet microtubules that generate the sliding force
in motile axonemes and control the flexion are all apparently absent in nematode
cilia.
The absence of basal bodies seems a paradox as they are believed to have two
functions, one of which is essential. First, they are the templates for the
ninefold structure of the axonemes. The nine doublet microtubules of the axoneme
are a direct extension of the A and B subfibers of the nine triplet-microtubules
in the basal body. Second, basal bodies are attachment points for cytoplasmic
microtubules which anchor the cilium to the cytoskeleton. This coupling is
essential for transmitting force from a beating cilium into cell motion. It may
also be useful for holding cilia erect from the cell surface.
In nematodes, the mechanical role of the basal body is probably not needed. The
template role would be filled if the centriole is present only transiently to
initiate the cilium and then disappears. Alternatively, the transitional fibers
themselves could be the residue of the centriole. Importantly, nematode
centrioles are composed of singlet microtubules, plus some attachments that may
be vestiges of B and C subfibers, rather than triplets (D. Albertson, A.
Crowther, and J. N. Thomson, personal communication). Finally, in view of these
departures from what are usually regarded as universal characteristics of
centrioles and cilia, it is worth mentioning that microtubules themselves may be
unusual in nematodes. Cytoplasmic microtubules in nerve processes contain only
11 protofilaments rather than the more usual 13 protofilaments (Chalfie and
Thomson, 1982).
Acknowledgments
Our many colleagues who generously provided strains are mentioned under
Materials and Methods. We thank R. Ware for sharing his unpublished observations
on the sensilla of chemosensory and thermo-sensory mutants; J. Weiss for
illustrations; and E. Aamodt, P. Albert, D. Albertson, A. Burr, M. Chalfie, D.
Dusenbery, L. Gremke, D. Hall, R. Herman, J. Hodgkin, C. Kenyon, B. Menco, D.
Riddle, R. Russell, S. Siddiqui, J. Sulston, S. Ward, J. White, and K. Wright
for ideas and discussions. In sadness, we acknowledge the assistance and
kindness of Kay Buck who died unexpectedly during the course of this work. Part
of this research was supported by a Basil O'Connor starter grant from the March
of Dimes Foundation and by NIH Grants NS16510 and NS20258 to J.C. E.H. was
recipient of postdoctoral fellowships from the Muscular Dystrophy Association of
America and the NIH.
References
APZELIUS, B. A. (1976). A human syndrome caused by immotile cilia.
Science 193, 317-319.
AFZELIUS, B. A. (1981). Genetic disorders of cilia. In
"International Cell Biology 1980-1981" (H. G. Schweiger, ed.), p. 440. Springer-Verlag, Berlin.
ALBERT, P. S., BROWN, S., and RIDDLE, D. L. (1981). Sensory
control of dauer larva formation in Caenorhabditis elegans. J. Comp. Neurol 198, 435-451.
BRENNER, S. (1974). The genetics of Caenorhabditis elegans.
Genetics 77, 71-94.
BURR, A. H. (1985). The photomovement of Caenorhabditis elegans. A
nematode which lacks ocelli. Proof that the response is to light not radiant heating. Photochem. Photobiol 41, 577-582.
CASSADA, R. C., and
RUSSELL, R. L. (1975). The dauerlarva, a post-embryonic developmental variant
of the nematode Caenorhabditis
elegans. Den. Biol 46, 326-342.
CHALFIE, M., and SULSTON, J. (1981).
Developmental genetics of the mechanosensory neurons of Caenorhabditis elegans. Dev. Biol. 82, 358-370.
CHALFIE, M., and THOMSON, J. N. (1982). Structural and functional diversity in the neuronal microtubules of
Caenorhabditis elegans. J. Cell Biol 93,15-23.
CULOTTI, J. G., and RUSSELL, R. L. (1978). Osmotic
avoidance defective
mutants of the nematode Caenorhabditis elegans. Genetics 90, 243-256.
DUSENBERY, D. B. (1974). Analysis of chemotaxis in the nematode
Caenorhabditis elegans by countercurrent separation. J. Exp. Zool. 188, 41-47.
DUSENBERY, D. B. (1975). Avoidance of D-tryptophan by the nematode
Caenorhabditis elegans. J. Exp. Zool. 193, 413-418.
DUSENBERY, D. B. (1976a). Attraction of the nematode Caenorhabditis elegans to
pyridine. Comp. Biochem. Physiol C 53, 1-2.
DUSENBERY, D. B. (1976b). Chemotactic behavior of mutants of the nematode
Caenorhabditis elegans that are defective in their attraction to NaCl. J. Exp. Zool 198, 343-352.
DUSENBERY, D. B. (1980a). Appetitive response of the nematode
Caenorhabditis
elegans to oxygen. J. Comp. Physiol 136, 333-336.
DUSENBERY, D. B. (1980b). Chemotactic behavior of mutants of the nematode
Caenorhabditis elegans that are defective in osmotic avoidance. J. Comp. Physiol 137, 93-96.
DUSENBERY, D. B. (1980c). Behavior of free-living nematodes. In "Nematodes as
Biological Models," (B. M. Zuckerman, ed.), Vol. 1, pp. 127-158. Academic
Press, New York.
DUSENBERY, D. B., and BARR, J. (1980). Thermal limits and chemotaxis in
mutants of the nematode Caenorhabditis elegans defective in thermotaxis. J.
Comp. Physiol 137, 353-356.
DUSENBERY, D. B., SHERIDAN, R. E., and RUSSELL, R. L. (1975).
Chemotaxis-defective mutants of the nematode Caenorhabditis elegans.
Genetics 80, 297-309.
FRAENKEL, G. S., and GUNN, D. L. (1961). The Orientation of Animals. Dover,
New York.
GILULA, N. B., and SATIR, P. (1972). The ciliary necklace: A ciliary membrane
specialization. J. Cell Biol 53, 494-509.
GOLDEN, J. W., and RIDDLE, D. L. (1982). A pheromone influences larval
development in the nematode Caenorhabditis elegans. Science 218, 578-580.
GOLDEN, J. W., and RIDDLE, D. L. (1984a). A pheromone-induced developmental
switch in Caenorhabditis elegans: Temperature-sensitive mutants reveal a
wild-type temperature-dependent process. Proc. Natl Acad. Sci. USA 81,
819-823.
GOLDEN, J. W., and RIDDLE, D. L. (1984b). The Caenorhabditis elegans dauer
larvae: Developmental effects of pheromone, food, and temperature. Dev. Biol.
102, 368-378.
GOODENOUGH, U. W., and ST. CLAIR, H. S. (1975). Bald-2: A mutation affecting
the formation of doublet and triplet sets of microtubules in Chlamydomonas
reinhardtii. J. Cell Biol 66, 480-491.
HALL, D. H., and RUSSELL, R. L. (1986). Electron microscopic reconstruction of
the posterior nervous system of the nematode Caenorhabditis elegans. Philos.
Trans. R. Soc., in press.
HEDGECOCK, E. M., and RUSSELL, R. L. (1975). Normal and mutant thermotaxis in
the nematode Caenorhabditis elegans. Proc. Natl Acad. Sci USA 72, 4061-4065.
HEDGECOCK, E. M., CULOTTI, J. G., THOMSON, J. N., and PERKINS, L. A. (1985).
Axonal guidance mutants of Caenorhabditis elegans identified by filling
sensory neurons with fluorescein dyes. Dev. Biol 111, 158-170.
HERMAN, R. K. (1984). Analysis of genetic mosaics in the nematode Caenorhabditis elegans. Genetics 108,165-180.
HODGKIN, J. (1983). Male phenotypes and mating efficiency in
Caenorhabditis
elegans. Genetics 103, 43-64.
HODGKIN, J., HORVITZ, H. R., and BRENNER, S. (1979). Nondisjunction mutants of
the nematode Caenorhabditis elegans. Genetics 91, 67-94.
HORVITZ, H. R., CHALFIE, M., TRENT, C., SULSTON, J. E. and EVANS, P. D.
(1982). Serotonin and octopamine in the nematode Caenorhabditis elegans.
Science 216, 1012-1014.
KUNG, C., CHANG, S.-Y., SATOW, Y., VAN HOUTEN, J., and HANSMA,
H. (1975). Genetic dissection of behavior in Paramecium. Science 188,
898-904.
LEWIS, J. A. and HODGKIN, J. A. (1977). Specific neuroanatomical
changes in chemosensory mutants of the nematode Caenorhabditis
elegans. J. Comp. Neurol 172, 489-509.
LUCK, D. J. L. (1984). Genetic and
biochemical dissection of the eukaryotic flagellum. J. Cell Biol 98, 789-794.
RAND, J. B., and RUSSELL, R. L.
(1984). Choline acetyltransferase-deficient mutants of the nematode Caenorhabditis elegans. Genetics
106, 227-248.
REESE, T. S. (1965). Olfactory cilia in the frog. J. Cell Biol 25,209-230.
RIDDLE, D. L., SWANSON, M. M., and ALBERT, P. S. (1981). Interacting
genes in nematode dauer larva formation. Nature (London) 290,668-671.
RINGO, D. D. (1967). Flagellar motion and fine structure of the
flagellar apparatus in Chlamydomonas. J. Cell Biol 33, 543-571.
SALISBURY, J. L., and
FLOYD, G. L. (1978). Calcium-induced contraction
of the rhizoplast of a quadrilflagellate green alga. Science 202, 975-977.
SALISBURY, J. L., BARON, A., SUREK, B., and MELKONIAN, M. (1984).
Striated flagellar roots: Isolation and partial characterization of a calcium-modulated
contractile organelle. J. Cell Biol 99, 962-970.
SULSTON,
J., DEW, M., and BRENNER, S. (1975). Dopaminergic neurons
in the nematode Caenorhabditis elegans. J. Comp. Neurol. 163, 215-226.
SULSTON, J. E., and HORVITZ, H. R. (1977). Postembryonic cell lineages
of the nematode Caenorhabditis elegans. Dev. Biol 56,110-156.
SULSTON, J. E.,
and WHITE, J. G. (1980). Regulation and cell autonomy
during postembryonic development of Caenorhabditis elegans. Dev.
Biol 78, 577-597.
SULSTON, J. E., ALBERTSON, D. G., and THOMSON, J. N. (1980). The
Caenorhabditis elegans male: Postembryonic development of nongonadal
structures. Dev. Biol 78, 542-576.
SULSTON, J. E., SCHIERENBERG, E., WHITE, J.
G., and THOMSON, J. N.(1983). The embryonic cell lineage of the nematode Caenorhabditis
elegans. Dev. Biol. 100, 64-119.
SWANSON, M. M., EDGLEY, M. L., and RIDDLE, D.
L. (1984). Genetic
Maps 3, 286-299.
THURM, U., ERLER, G., GODDE, J., KASTRUP, H., KEIL, TH.,
VVOLKER,W., and VOHWINKEL, B. (1983). Cilia specialized for mechanoreception. J. Submicrosc. Cytol 15, 151-155.
TRENT, C., TSUNG, N., and HORVITZ, H.
R. (1983). Egg-laying defective
mutants of the nematode Caenorhabditis elegans. Genetics 104,619-647.
WARD, S. (1973). Chemotaxis by the nematode Caenorhabditis elegans:Identification of attractants and analysis of the response by use of
mutants. Proc. Natl Acad. Sci. USA 70, 817-821.
WARD, S., THOMSON, N., WHITE,
J. G. and BRENNER, S. (1975). Electron
microscopic reconstruction of the anterior sensory anatomy of the
nematode Caenorhabditis elegans. J. Comp. Neurol 160, 313-338.
WARD, S.,
ROBERTS, T. M., NELSON, G. A., and ARGON, Y. (1982). The
development and motility of Caenorhabditis elegans spermatozoa. J.
Nematol. 14, 259-166.
WARE, R. W., CLARK, D., CROSSLAND, K., and RUSSELL, R.
L. (1975).The nerve ring of the nematode Caenorhabditis elegans: Sensory input
and motor output. J. Comp. Neurol 162, 71-110.
WHITE, J. G., SOUTHGATE, E.,
THOMSON, J. N., and BRENNER, S. (1986).
The nervous system of Caenorhabditis elegans. Philos. Trans. R. Soc.,
in press.
WRIGHT, K. A. (1980). Nematode sense organs. In "Nematodes as Biological
Models." (B. M. Zuckerman, ed.), Vol. 2, pp. 237-295. Academic
Press, NewYork.
WRIGHT, K. A. (1983). Nematode chemosensilla; form and
function. J.Nematol 15,151-158.
WRIGHT, R. L., CHOJNACKI, B., and JARVIK, J. W. (1983). Abnormal
basal-body number, location, and orientation in a striated fiber-defective mutant
of Chlamydomonas reinhardtii. J. Cell Biol 96,1697-1707.
|