Mounting Animals for Observation with Nomarski DIC Optics: by Monica Driscoll
PDF version of this protocol
1 Preparation of Agar Pads:
Materials
5% agar solution in water, melted and kept molten by placing the tube in a heat block at 65°C
Pasteur pipet and bulb
2 glass slides with pieces of labeling tape (for example, Fisher #11-880-5-D) taped over both ends to serve as spacers
2 clean glass slides
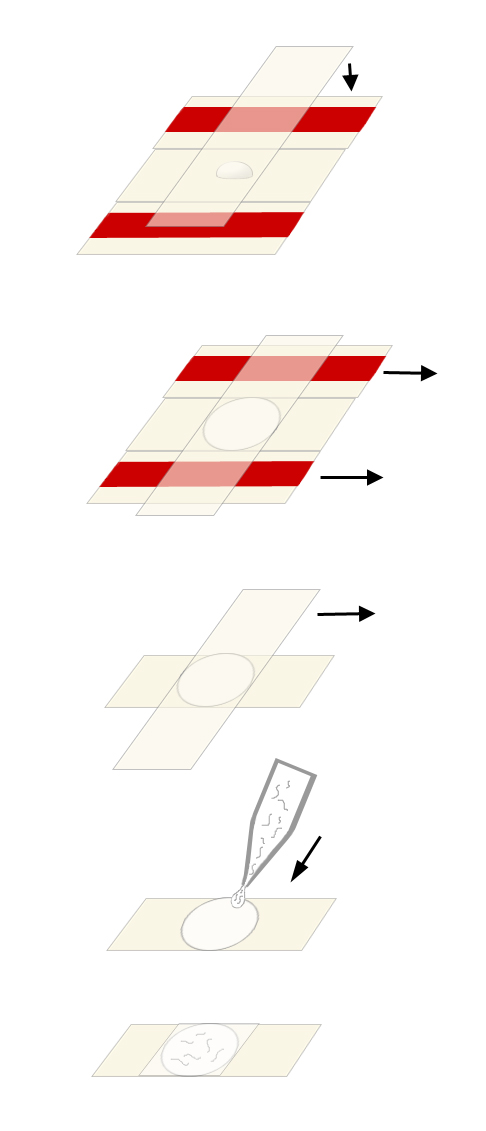
Preparation of Pads (See Figure below):
Place the two taped slides with a clean slide sandwiched between them on a flat surface.
Using the Pasteur pipet, place a drop of agar onto the clean slide.
Cover the agar with another clean slide placed on top of the three slides in a perpendicular fashion. Press gently so the agar drop is flattened to a circle about 0.4 mm thick (the thickness of the tape spacers). Avoid getting bubbles in the agar since worms will get stuck in them.
After the agar solidifies, first gently pull out the taped slides, then gently separate the remaining two slides so that the agar pad adheres to one of them (usually the bottom one). Rest the slide agar side-up on the bench top.
Note: The agar pad should be prepared just before use so that it does not dry out. Alternatively, once you pull out the taped slides, you can wrap the cross shaped slide-agar-slide in a piece of Saran wrap. This way you can keep the pad moist for a couple of hours. When you get more experienced and know how much pressure is needed to get the appropriate thickness of pad, you can skip the taped slides and simply stack clean slides on top of each other with agar drops sandwiched between them.
2 Mounting Live Animals:
Place 1-2 ul drop of M9 containing 10-25 mM sodium Azide (NaN3) onto the center of the agar pad. NaN3 anesthetizes the worms so that they will not move.
Transfer animals to be observed into the drop. Animals can be transferred using a worm pick or an eye brow hair fastened to a toothpick with wood glue or clear nail polish. getting animals or eggs stick to the eyebrow is a skill acquired with practice. When the hair or pick is moved into the NaN3 drop, animals float off easily. Alternatively, animals to be viewed can be spun down in M9 in an eppendorf tube at 1200-1500 rpm with slow acceleration/deceleration. After the supernatant is discarded, the animals are suspended in 10 µl M9-NaN3. 2-3 µl of the solution with the animals can then be transferred to the pad with an Eppendorf pipet. For easy transfer and avoiding animals to stick to the tip, the pipet tip should be cut to a larger bore. Another alternative is to prepare the agar pad with anesthetic included for a final concentration of 10 mM NaN3 in the agar, instead of having it in the solution.
Gently lay a coverslip over the animals. Most animals will lie on their sides.
|