|
The Structure of the Ventral Nerve Cord
of Caenorhabditis elegans
J.G. White, Eileen Southgate, J.N. Thomson and S. Brenner F.R.S.
Phil. Trans. Royal Soc. London (1976) 275B(938): 327-348
doi: 10.1098/rstb.1976.0086
(Communicated by S. Brenner, F.R.S. Received 15 September 1975)
Abstract - Introduction -
Material & Methods -
Discussion -
Acknowledgments
- References
Abstract
The nervous system of Caenorhabditis elegans is arranged as a series of fibre bundles which run along internal hypodermal ridges. Most of the sensory integration takes place in a ring of nerve fibres which is wrapped round the pharynx in the head. The body muscles in the head are innervated by motor neurones in this nerve ring while those in the lower part of the body are innervated by a set of motor neurones in a longitudinal fibre bundle which joins the nerve ring, the ventral cord. These motor neurones can be put into five classes on the basis of their morphology and synaptic input. At any one point along the cord only one member from each class has neuromuscular junctions. Members of a given class are arranged in a regular linear sequence in the cord and have non-overlapping fields of motor synaptic activity, the transition between fields of adjacent neurones being sharp and well defined. Members of a given class form gap junctions with neighbouring members of the same class but never to motor neurones of another class. Three of the motor neurone classes receive their synaptic input from a set of interneurones coming from the nerve ring. These interneurones can in turn be grouped into four classes and each of the three motor neurone classes receives its synaptic input from a unique combination of interneurone classes. The possible developmental and functional significance of these observations is discussed.
Introduction
One approach to the study of the function, development and genetic specification of nervous systems is to use an organism that is both amenable to genetic analysis and has a simple nervous system that may be completely characterized anatomically. The analysis of any structural alterations in behavioural mutants might provide some insight into both the functioning of the nervous system and the execution of the developmental program constructing it. The nematode Caenorhabditis elegans has been chosen as a suitable organism for this type of study and many behavioural mutants have been isolated and mapped (Brenner 1974). This paper is the second of a series on the structure of the nervous system of the wild type animals (Ward, Thornson, White & Brenner 1975). It describes the organization of the neuromuscular system mediating locomotion as determined by reconstructions from serial section electron micrographs.
Materials & Methods
(a) General anatomy
(b) The hypodermis and cuticle
(c) Body muscles
(d) The ventral and dorsal nerve cords
(e) Interneurones
(f) Motor neurones
(g) Summary of neuromuscular circuitry
(h) Invariance
(i) Synaptic variability
(ii) Morphological invariance
Cultures of C. elegans were grown in petri dishes containing a bacterial lawn on an agar substrate (Brenner 1974). Individual adult hermaphrodites were fixed, sectioned and stained for electron microscopy as described by Ward et al. (1975). Sections were photographed in the electron microscope at a primary magnification of 7500 and printed. Individual nerve processes were followed through on the prints and labelled. Use was also made of a computer aided reconstruction system. Electron micrographs were transcribed in relative alignment onto 35 mm film. This was then back projected onto a coordinate digitizer by means of a computer controlled projector. The digitizer was interfaced to a Modular One computer and outlines of nerve processes were drawn on it and stored by the computer on magnetic disk. The outlines were also displayed on a Cossor C.S.D. 1000 graphics terminal interfaced to the computer and could be edited by means of commands typed in on the terminal's keyboard. As sections were traced notes were taken on the various features that were seen and this information was subsequently entered into the computer and merged with the coordinate data to make a data base containing all the information that had been abstracted from a particular series. Various types of information could be abstracted from this data base and displayed on the graphics terminals, such as three dimensional reconstructions, branching topologies and circuit diagrams. This system, which is similar to that of Levinthal & Ware (1972), has been more fully described by White (1974).
The structures that are to be described were obtained from four reconstructed series of adult hermaphrodites designated 0, S, T and U. The longest of these was the U series of about 6000 sections, which began at the head, included the nerve ring and anterior half of the ventral cord, and went as far back as the vulva. When the reconstructions were being done arbitrary labels were given to the cells in the various series. These labels were subsequently normalized to those used in the U series when the reconstructions were completed and the equivalent cells in the different series identified. These are the labels that are used in the text.
Two types of specialized contact sites between neurones could be readily identified and scored; chemical synapses and gap junctions. The former are generally in the form of a varicosity along the length of a process containing an aggregation of vesicles adjacent to a convex, electron dense membrane specialization (figure 1, plate 1). There is usually only one obvious postsynaptic cell but sometimes there are two or more resembling the dyads and triads described by Dowling & Boycott (1966). There are no visible specializations in the membranes of the post-synaptic cells. The vesicles associated with all synapses that will be described in the ventral cord are spherical with a mean diameter of 40 nm and all have similar staining properties. Other types of vesicle have been described in the nerve ring by Ware, Clark, Crossland & Russell (1975). Gap junctions are identified as regions where the cell membranes from two adjacent cells are in close apposition and become more electron dense (figure 2, plate 1). There is a nonstaining intercellular cleft 8 nm wide separating the two membranes. The region of contact is usually straight or has a slight curvature whereas the membranes outside this region are more convoluted. These junctions closely resemble those described by Revel & Karnovsky (1967). All gap junctions and chemical synapses referred to in the body of this paper were assigned by using the above criteria.
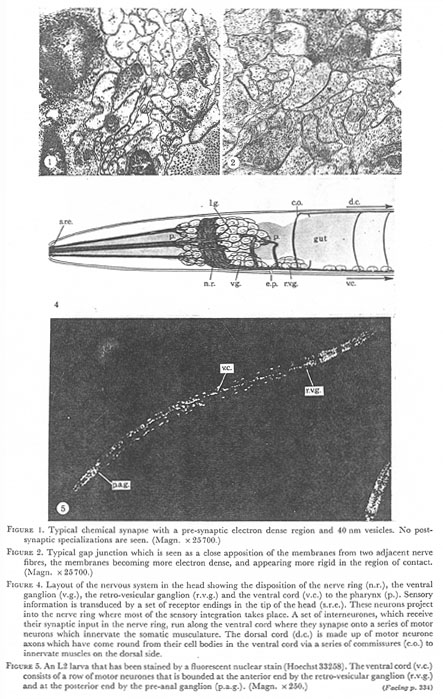 Figures 1, 2, 4, 5. Plate 1.
Figures 1, 2, 4, 5. Plate 1.
Figure 1. Typical chemical synapse with a pre-synaptic electron dense region and 40 nm vesicles. No post-synaptic specializations are seen. (Magn. x 25700.)
Figure 2. Typical gap junctions which is seen as a close a apposition of the membranes from two adjacent nerve fibers, the membranes becoming more electron dense, and appearing more rigid in the region of contact. (Magn. x 25700.)
Figure 4. Layout of the nervous system in the head showing the disposition of the nerve ring (n.r.), the ventral ganglion (v.g.), the retro-vesicular ganglion (r.v.g.) and the ventral cord (v.c.) to the pharynx (p.). Sensory information is transduced by a set of receptor endings in the tip of the head (s.r.e.). These neurons project into the nerve ring where most of the sensory integration takes place. A set of interneurones, which receive their synaptic input in the nerve ring, run along the ventral cord where they synapse onto a series of motor neurons which innervate the somatic musculature. The dorsal cord (d.c.) is made up of motor neurone axons which have come round from their cell bodies in the ventral cord via a series of commissures (c.o.) to innervate muscles on the dorsal side.
Figure 5. An L2 larva that has been stained by a fluorescent nuclear stain (Hoechst33258). The ventral cord (v.c.) consists of a row of motor neurones that is bounded at the anterior end by the retro-vesicular ganglion (r.v.g.) and at the posterior end by the pre-anal ganglion (p.a.g.). (Magn. x 250.)
(a) General anatomy
The animal is ensheathed in a tough, impenetrable elastic cuticle which is laid down by a system of hypodermal cells underlying it. The body cavity (the pseudocoelom) is maintained at a high hydrostatic pressure relative to the outside and it is this pressure acting on the elastic cuticle which gives it rigidity and structural integrity (the so-called hydrostatic skeleton Crofton 1966).
Body movements are mediated by four strips of muscle cells running longitudinally in four quadrants. There are about 24 cells in each quadrant of the adult and these are packed diagonally in two rows (figure 3). The muscle cells lie directly underneath the hypodermis and elastically deform the cuticle against the stress produced by the turgor pressure. Nematode muscles are unusual in that they send arms which go out to motor neurones to receive innervation rather than the motor neurones coming to the muscle (Schneider 1860).
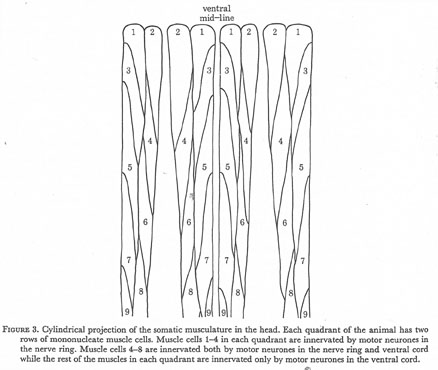 Figure 3. Cylindrical projection of the somatic musculature in the head. Each quadrant of the animal has two rows of mononucleate muscle cells. Muscle cells 1-4 in each quadrant are innervated by motor neurones in the nerve ring. Muscle cells 4-8 are innervated both by motor neurones in the nerve ring and ventral cord while the rest of the muscles in each quadrant are innervated only by motor neurones in the ventral cord.
Figure 3. Cylindrical projection of the somatic musculature in the head. Each quadrant of the animal has two rows of mononucleate muscle cells. Muscle cells 1-4 in each quadrant are innervated by motor neurones in the nerve ring. Muscle cells 4-8 are innervated both by motor neurones in the nerve ring and ventral cord while the rest of the muscles in each quadrant are innervated only by motor neurones in the ventral cord.
Most of the nematode's nervous system is situated in the head and is organized around a large pharynx which is a virtually self-contained neuromuscular system used to pump food into the gut and maintain the turgor pressure (Albertson & Thomson 1976). The head is richly endowed with sensory receptors, most of which have their endings near the mouth (Ward et al. 1975). There are two main classes of receptor in the head; one is generally considered to be mechanosensory and consists of two concentric rings of sensilla projecting back to cell bodies which are predominantly situated in front of the nerve ring. The other class of receptors is arranged in two lateral sensilla, the amphids, and these are generally taken to be chemosensory. The neurones in these sensilla project back to cell bodies that are in the lateral ganglia behind the ring. The cell bodies for the sensory receptors, together with interneurone and some motor neurone cell bodies are situated between the two bulbs of the pharynx (figure 4, plate 1). These cells send out processes which run circumferentially round the pharynx as a fibre bundle forming the nerve ring, which is the major region of neuropile in the animal. A large proportion of the processes in the ring enter and leave on the ventral side forming the ventral nerve cord. This is initially quite large being composed predominantly of processes that run into the ring from the ventral ganglion and processes which enter the ventral cord via commissures from the cells situated in the posterior part of the lateral ganglia (figures 4 and 5, plate 1). Further down the body behind the excretory pore the cord is smaller and consists of two types of interneurones; one connects with the ganglia in the tail and has very few synapses in the cord and the other synapses onto a linear sequence of motor neurones which innervate the body musculature. The head muscles receive their innervation from motor neurone axones which lie on the inside of the nerve ring. Some of these motor neurones are also sensory receptors in the tip of the head (Ward et al. 1975).
The following sections describe the organization of the hypodermis and muscle in the body and the structure of the nervous circuitry of the ventral cord.
(b) The hypodermis and cuticle
The hypodermis consists of a system of cells which are joined so as completely to envelop the animal. Its prime function is that of secreting the cuticle which is a multi-layered structure composed mainly of modified forms of collagen (Bird 1971). Three layers can be seen in the cuticle of the adults (figure 8, plate 3), the outside one being separated from the inner two by a space with occasional localized attachment points joining them. The hypodermal cell bodies lie in four internal ridges which run along the lateral, dorsal and ventral lines. The body muscles lie in four quadrants between these ridges which are joined by thin sheets (0.2 µm thick) of hypodermis underlying the muscles.
Hypodermal nuclei are present in the two lateral, dorsal and ventral ridges in the head but are absent from the dorsal ridge in the body behind the nerve ring. Nerve processes generally run in close apposition to hypodermal ridges. A typical arrangement is shown in figure 6, plate 2; a bundle of nerve fibres runs adjacent to a ridge of hypodermis and both are surrounded by a basement membrane of 10 nm thickness. This membrane is often seen to have striations with a spacing of 30 nm which run in parallel with the nerve fibres (figure 9, plate 3). Nerve fibres lie under the basement membrane which separates the nervous and hypodermal tissues from the muscle, gut and gonad. Neuromuscular junctions occur through the membrane with the motor neurones and muscle arms always staying on opposite sides. The dorsal nerve cord (figure 7, plate 2) is largely made up of axons of motor neurones which have their cell bodies in the ventral cord and run round the outside of the animal between the hypodermis and the basement membrane as a circumferential commissure (figure 6, plate 2). When these processes reach the dorsal hypodermal ridge they turn and run along the dorsal nerve cord in an anterior or posterior direction depending on cell class. Commissures generally leave the ventral nerve cord on the right hand side but in some regions they leave on the left. Although the pattern is always the same. Both these routes are equivalent, leading eventually to the dorsal cord, and there seems to be no other differences in neurones which take the left hand route.
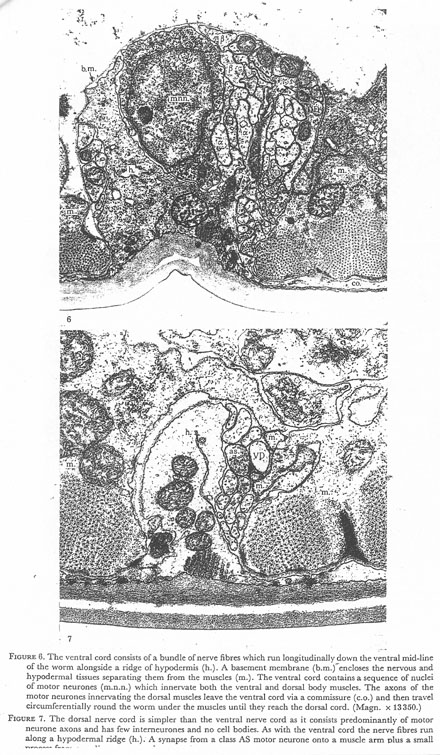 Figures 6 and 7. Plate 2.
Figures 6 and 7. Plate 2.
Figure 6. The ventral cord consists of a bundle of nerve fibres which run longitudinally down the ventral mid line of the worm alongside a ridge of hypodermis (h.). A basement membrane (b.m.) encloses the nervous and hypodermal tissues separating them from the muscles (m.). The ventral cord contains a sequence of nuclei of motor neurones (m.n.n.) which innervate both the ventral and dorsal body muscles. The axons of the motor neurons innervating the dorsal muscles leave the ventral cord via a commissure (c.o.) and then travel circumferentially round the worm under the muscles until they reach the dorsal cord. (Magn. x 13350.)
Figure 7. The dorsal nerve cord is simpler than the ventral nerve cord as it consists predominantly of motor neurone axons and has few interneurones and no cell bodies. As with the ventral cord the nerve fibres run along a hypodermal ridge (h.). A synapse from a class AS motor neurone onto a muscle arm plus a small process from an adjacent arm and a ventral class D (VD) dendrite is shown. (Magn. x 28300.)
(c) Body muscles
There are 95 mononucleate muscle cells in the hermaphrodite which are used for locomotion (R. Horvitz, personal communication). They are arranged in four quadrants each containing two rows of cells which are packed diagonally (figure 3). The contractile filaments of the muscle cells are arranged longitudinally and are inclined at only a few degrees from the Z lines which also run longitudinally rather than being normal to the filaments as in the case of conventional striated muscle. For this reason nematode muscle is referred to as being obliquely striated (Rosenbluth 1965). Each quadrant generally contains 12 sarcomeres in the adult. There are no specialized attachment points at the ends of the muscles but periodic electron dense regions are seen in the Z bands close to the cell wall which may well be the regions where the muscle is attached to the underlying hypodermis (figure 8, plate 3). The body muscles (cells 9-24) are innervated by motor neurones in the dorsal and ventral nerve cords. The muscles in the two subventral quadrants send out arms which branch and interdigitate with each other as they come into close apposition to the motor neurones in the ventral cord. The muscles in the two subdorsal quadrants behave in the same way being innervated by a common set of motor neurones in the dorsal cord. Thus, on both the dorsal and ventral side, right and left muscle cells receive a common synaptic input and so probably cannot operate independently. This is consistent with the observation that the worms can only bend their bodies in the dorsoventral plane (Croll 1970).
The first eight muscles in each quadrant of the head (1-8) send out arms which run posteriorly past the nerve ring and then turn and run anteriorly to receive their synaptic input from motor neurones lying on the inner surface of the nerve ring. Muscle arms from each quadrant are subdivided into those from the medial and those from the lateral row of muscles. There are eight distinct neuromuscular junction regions within the ring, one for each row of muscle cells. Motor neurones generally span two of these regions, either two adjacent medial or lateral regions or a pair from a particular quadrant. Pairs of adjacent muscle rows can therefore be activated independently giving the head an extra degree of freedom of movement over the body.
Muscle cells 5, 6, 7 and 8 in each quadrant are dually innervated, sending out arms to the ventral and dorsal nerve cords as well as into the ring (Ware et al. 1975). These muscles may therefore be driven either by the cord logic, when presumably they will be used to propagate waves of muscle contraction used for locomotion, or by the ring logic when they may be used for the head searching movements which usually occur when the body is stationary and the animal is feeding.
Gap junctions are seen between muscle cells and these occur both between muscle arms (figure 10, plate 3) and muscle cell bellies (figure 11, plate 3). De Bell, del Castillo & Sanchez (1963)found that the muscle cells in Ascaris lumbricoides are coupled together electrically and so it seems likely that this is also the case with C. elegans the electrical coupling being mediated by these gap junctions.
The muscle arms from the first four muscle cells in each quadrant (i.e. the cells that are not also innervated in the ventral cord) are far more extensive in the ring than those from the dually innervated muscles (i.e. muscles 5-8 in each quadrant). They also form gap junctions between their arms whereas the others do not. These gap junctions are between the same numbered cells but in adjacent quadrants therefore coupling two adjacent medial rows together and also two adjacent lateral rows.
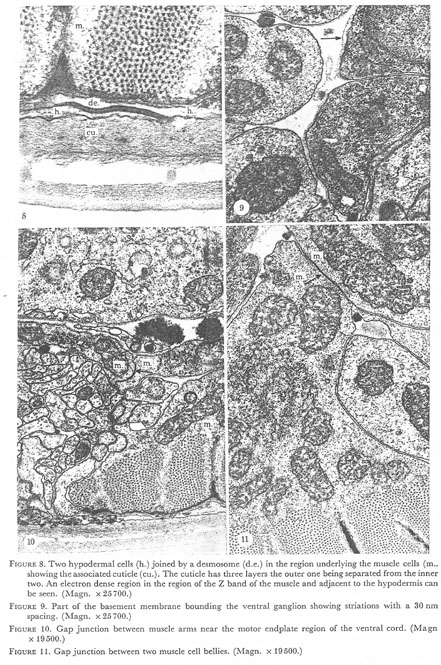 Figures 8-11. Plate 3.
Figures 8-11. Plate 3.
Figure 8. Two hypodermal cells (h.) joined by a desmosome (d.e.) in the region underlying the muscle cells (m.) showing the associated cuticle (cu.). The cuticle has three layers the outer one being separated from the inner two. An electron dense region in the region of the Z band of the muscle and adjacent to the hypodermis can be seen. (Magn. x 25700.)
Figure 9. Part of the basement membrane bounding the ventral ganglion showing striations with a 30 nm spacing. (Magn. x 25700.)
Figure 10. Gap junction between muscle arms near the motor endplate region of the ventral cord. (Magn. x 19500.)
Figure 11. Gap junction between two muscle cell bellies. (Magn. x 19500.)
(d) The ventral and dorsal nerve cords
The ventral cord contains a linear sequence of 57 motor neurones (figure 5, plate 1). These motor neurones innervate the body muscles on both the ventral and dorsal sides. The ventral motor neurones have axons which run along the right hand side of the ventral cord and synapse onto muscle arms from the two subventral quadrants of muscle (figures 12&13, 14&15 and 16&17, plates 4-6). Motor neurone axons are unbranched and generally form neuromuscular junctions (n.m.js) only over a well-defined region of the axon. Motor neurones innervating the two sub-dorsal quadrants of muscle send out processes which leave the ventral cord (figure 6, plate 2) and run round the outside of the animal as a commissure until they reach the dorsal hypodermal ridge when they turn and form the dorsal cord, innervating the dorsal muscles in the same way as the ventral muscles (figure 7, plate 2). The dorsal cord is predominantly made up of these motor neurone axons whereas the ventral cord also contains groups of processes from interneurones.
The anterior end of the ventral cord runs into the retrovesicular ganglion (r.v.g.), a small ganglion of 20 cells which is located just posterior to the excretory pore (figure 4, plate 1). Half the cells in the r.v.g. are motor neurones and are really logically part of the ventral cord. The posterior end of the cord runs into the pre-anal ganglion (p.a.g.) and although the cells of the p.a.g. have not yet been characterized it seems likely that some of them will also be ventral cord motor neurones.
The ventral cord is spatially coherent; neurones, particularly interneurones running in the cord, maintain their positions relative to their nearest neighbours in spite of local distortions produced by intrusions of cell bodies. The motor neurones which are driven by interneurones run in a region below the interneurones while they are receiving synapses but take up an adjacent position on the right hand edge of the nerve cord when they are synapsing onto muscles (figures 12&13, 14&15 and 16&17, plates 4-6). Motor neurones thus have clearly defined axonal and dendritic regions unlike most other neurones which both send and receive synapses en passant.
The motor neurones in the ventral cord together with the interneurones that synapse onto them can be grouped into distinct classes. These classifications are made on the basis of the topography of the cell and its synaptic circuitry. Either one of these criteria alone is sufficient to assign a particular neurone to a class. Neurones in a given class will always run in a fixed position in the nerve fibre bundle and usually run next to one another. Gap junctions are nearly always seen between adjacent members of a class and this can be considered a necessary condition for inclusion in a class. It is not, however a sufficient condition as gap junctions also occur between certain classes.
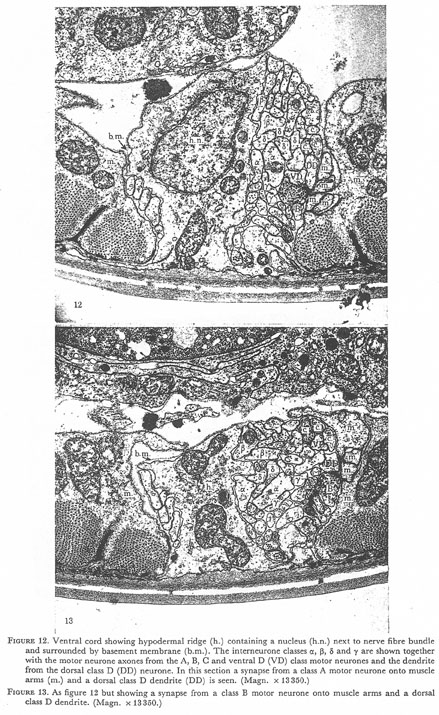 Figures 12 and 13. Plate 4.
Figures 12 and 13. Plate 4.
Figure 12. Ventral cord showing hypodermal ridge (h.) containing a nucleus (h.n.) next tot the nerve fibre bundle and surround by basement membrane (b.m.). The interneurone classes α, β, δ, and γ are shown together with the motor neurone axons from the A, B, C and ventral D (VD) class motor neurones and the dendrite from the dorsal class D (DD) neurone. In this section a synapse from a class A motor neurone onto muscle arms (m.) and dorsal class D dendrite (DD) is seen (Magn. x 13350.)
Figure 13. As figure 12 but showing a synapse from a class B motor neurone onto muscle arms and a dorsal class D dendrite. (Magn. x 13350.)
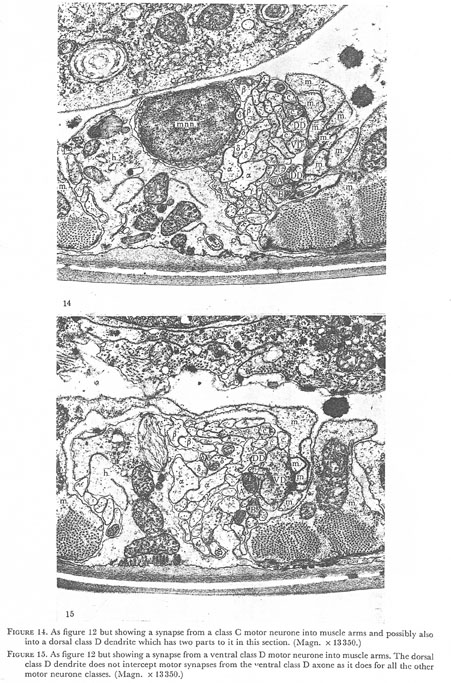 Figures 14 and 15. Plate 5.
Figures 14 and 15. Plate 5.
Figure 14. As figure 12 but showing a synapse from a class C motor neurone into muscle arms and possibly also into dorsal class D dendrite which has two parts to it in this section. (Magn. x 13350.)
Figure 15. As figure 12 but showing a synapse from a ventral class D motor neurone into the muscle arms. The dorsal class D dendrite does not intercept motor synapses from the ventral class D axone as it does for all the other motor neurone classes. (Magn. x 13350.)
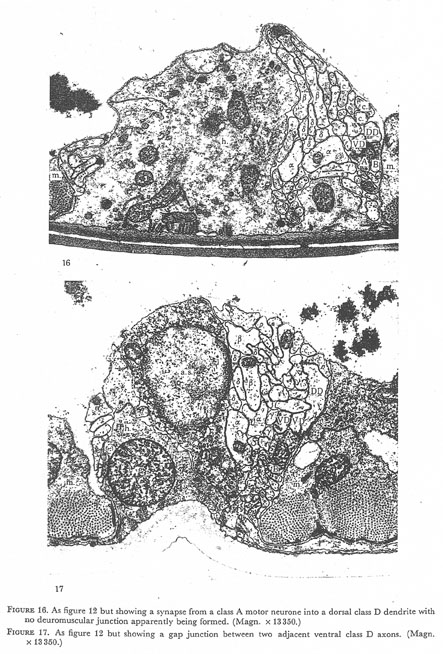 Figures 16 and 17. Plate 6.
Figures 16 and 17. Plate 6.
Figure 16. As figure 12 but showing a synapse from class A motor neurone into dorsal class D dendrite with no neuromuscular junction apparently being formed. (Magn. 13350.)
Figure 17. As figure 12 but showing a gap junction between two adjacent ventral class D axons (Magn. x 13350.)
(e) Interneurones
WA editor's (Z. Altun) note: Current nomenclature for the ventral nerve cord interneurones are; alpha=AVA, beta=AVB, delta=AVD and AVE and gamma=PVC.
There are four classes of interneurones in the set which innervate motor neurones in the ventral cord; these have been designated α, β, δ, and γ. The δ and β classes each have two members and are the most prominent interneurones in the cord. The β-neurones are a bilaterally symmetric pair of monopolar cells which lie in the posterior part of the lateral ganglia. They enter the ring via the ventral ganglia, run round the ring and then run down the ventral cord. The α-neurones are also a bilaterally symmetric pair of monopolar cells but they lie in the anterior part of the lateral ganglion and they enter the ring directly laterally and run round it leaving it to run down the ventral cord underneath the β-neurones (figure 12&13, 14&15 and 16&17, plates 4-6). The β-neurones form chemical synapses to the α-neurones along the length of the cord. The synapses are sparse in the anterior end of the cord but increase in density down the cord. The δ-neurones are similar in general layout to the alpha having cell bodies in the anterior part of the lateral ganglia and running round the ring before entering the cord. There are four δ-neurones, two bilaterally symmetrical pairs. Two of them (AVE) end in the anterior part of the cord but the other (AVD)along with the γ- and β-neurones, run past the vulva into the posterior region of the cord. The δ-interneurones form extensive synapses onto the α-neurones along the length of the cord and run between the α- and β-classes. The γ-interneurones are a bilaterally symmetric pair whose processes run underneath those from the α-neurones. They end in the nerve ring and form fewer connections in the anterior part of the cord compared with the other classes. The interneurones receive most of their synaptic input in the nerve ring; these connections will be described in detail in a subsequent paper.
(f) Motor neurones
WA editor's (Z. Altun) note: Current nomenclature for the ventral nerve cord motorneurones are; A=VA and DA, B=VB and DB, C=VC, D=VD and DD.
There are five classes of motor neurone in the ventral cord designated A, B, AS, C and D. The AS neurones only innervate the dorsal side whereas the C type neurones only innervate the ventral side. The other classes innervate both the dorsal and ventral sides. The members of each class form a repeating set down the cord and each member (apart from those in class C) has a clearly defined region where neuromuscular junctions are formed. The position along the axon where one motor neurone stops giving n.m.js is fairly precisely aligned with the position on the axon of the adjacent motor neurone of the same class where n.m.js start (figures 18 and 19). These transition points are not the same for the different classes.
Motor neurones run directly underneath the basement membrane in regions where they are forming n.m.js. They arrange themselves in a fixed order being on the ventral side from dorsal to ventral; class C, class D dendrite, class D axon, class A and class B (figures 12&13, 14&15 and 16&17, plates 4-6). The topography of the five classes of neurone are summarized in figure 20.
Type A motor neurones are bipolar cells which send axonal processes that run anteriorly forming neuromuscular connections. The dorsal type A cells have a process situated near to the cell body which runs round to the dorsal side as a commissure; there making an axonal branch that runs anteriorly. The ventral type A neurones have a dendritic branch (i.e. a branch that is exclusively post-synaptic) which courses posteriorly from the cell body, whereas the dorsal type A has a dendritic branch that runs anteriorly. They predominantly form gap junctions with α-interneurones but also receive chemical synapses from α, and δ-interneurones. Type A motor neurones form gap junctions with other adjacent type A and type AS neurones.
Type B motor neurones have the same morphology as type A except that they are inverted, i.e. the axonal branches project posteriorly but the dendritic arms run anteriorly in the ventral type B neurones and posteriorly in the dorsal. They predominantly form gap junctions with β-interneurones but also receive a few chemical synapses from γ-interneurones. They form gap junctions with adjacent type B cells.
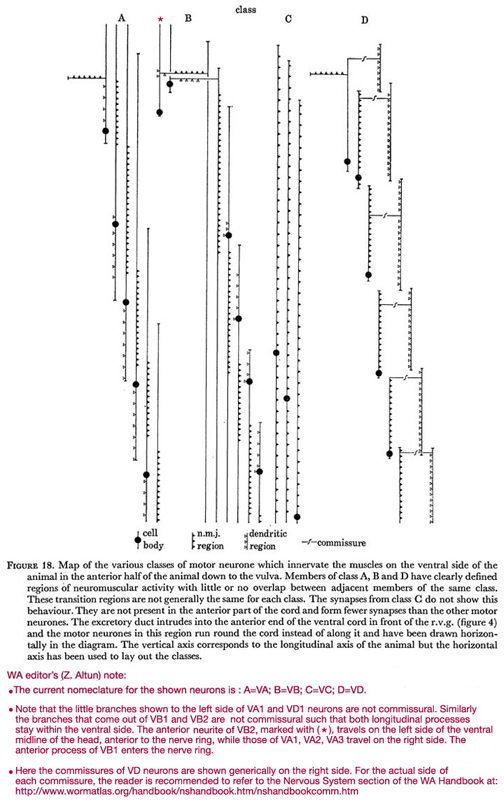 Figure 18. Map of the various classes of motor neurone which innervate the muscles on the ventral side of the animal in the anterior half of the animal down to the vulva. Members of class A, B and D have clearly defined regions of neuromuscular activity with little or no overlap between adjacent members of the same class. These transition regions are not generally the same for each class. The synapses from class C do not show this behaviour. They are not present in the anterior part of the cord and form fewer synapses than the other motor neurones. The excretory duct intrudes into the anterior end of the ventral cord in front of the r.v.g. (figure 4) and the motor neurones in this region run round the cord instead of along it and have been drawn horizontally in the diagram. The vertical axis corresponds to the longitudinal axis of the animal but the horizontal axis has been used to lay out the classes.
Figure 18. Map of the various classes of motor neurone which innervate the muscles on the ventral side of the animal in the anterior half of the animal down to the vulva. Members of class A, B and D have clearly defined regions of neuromuscular activity with little or no overlap between adjacent members of the same class. These transition regions are not generally the same for each class. The synapses from class C do not show this behaviour. They are not present in the anterior part of the cord and form fewer synapses than the other motor neurones. The excretory duct intrudes into the anterior end of the ventral cord in front of the r.v.g. (figure 4) and the motor neurones in this region run round the cord instead of along it and have been drawn horizontally in the diagram. The vertical axis corresponds to the longitudinal axis of the animal but the horizontal axis has been used to lay out the classes.
WA editor's (Z. Altun) note:
The current nomenclature for the shown neurons is: A=VA; B=VB; C=VC; D=VD.
Note that the little branches shown to the left side of VA1 and VD1 neurons are not commissural. Similarly the branches that come out of VB1 and VB2 are not commissural such that both longitudinal processes stay within the ventral side. The anterior neurite of VB2, marked with (*), travels on the left side of the ventral midline of the head, anterior to the nerve ring, while those of VA1, VA2, and VA3 travel on the right side. THe anterior process of VB1 enters the nerve ring.
Here are the commissures of VD neurones are shown generically on the right side. For the actual side of each commissure, the reader is recommended to refer to the Commissures section in the Nervous System Chapter of the WormAtlas Hermaphrodite Handbook.
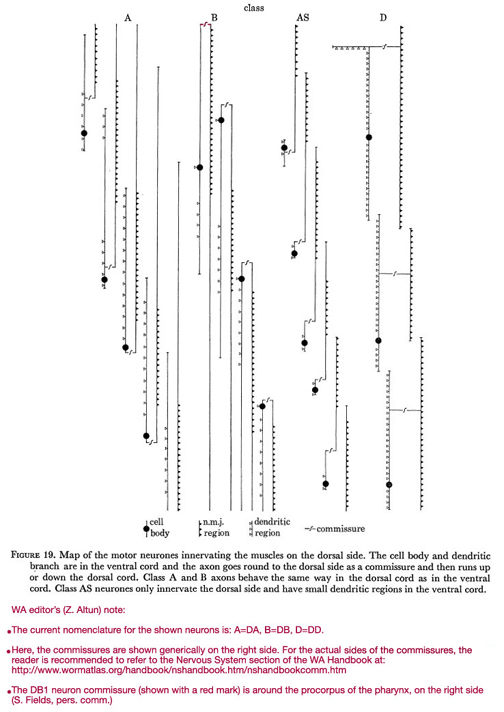 Figure 19. Map of the motor neurones innervating the muscles on the dorsal side. The cell body and dendritic branch are in the ventral cord and the axon goes round to the dorsal side as a commissure and then runs up or down the dorsal cord. Class A and B axons behave the same way in the dorsal cord as in the ventral cord. Class AS neurones only innervate the dorsal side and have small dendritic regions in the ventral cord.
Figure 19. Map of the motor neurones innervating the muscles on the dorsal side. The cell body and dendritic branch are in the ventral cord and the axon goes round to the dorsal side as a commissure and then runs up or down the dorsal cord. Class A and B axons behave the same way in the dorsal cord as in the ventral cord. Class AS neurones only innervate the dorsal side and have small dendritic regions in the ventral cord.
WA editor's (Z. Altun) note:
The current nomenclature for the shown neurons is: A=DA; B=DB; C=VC; D=DD.
Here are the commissures of VD neurones are shown generically on the right side. For the actual side of each commissure, the reader is recommended to refer to the Commissures section in the Nervous System Chapter of the WormAtlas Hermaphrodite Handbook.
The DB1 neuron commissure (shown with a red mark) is around the procorpus of the pharynx, on the right side (S. Fields, pers comm.).
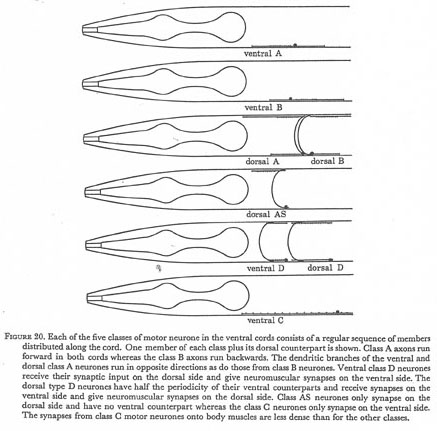 Figure 20. Each of the five classes of motor neurone in the ventral cords consists of a regular sequence of members distributed along the cord. One member of each class plus its dorsal counterpart is shown. Class A axons run forward in both cords whereas the class B axons run backwards. The dendritic branches of the ventral and dorsal class A neurones run in opposite directions as do those from class B neurones. Ventral class D neurons receive their synaptic input on the dorsal side and give neuromuscular synapses on the ventral side. The dorsal type D neurones have half the periodicity of their ventral counterparts and receive synapses on the ventral side and give neuromuscular synapses on the dorsal side. Class AS neurones only synapse on the dorsal side and have no ventral counterpart whereas the class C neurones only synapse on the ventral side. The synapses from class C motor neurones onto body muscles are less dense than for the other classes.
Figure 20. Each of the five classes of motor neurone in the ventral cords consists of a regular sequence of members distributed along the cord. One member of each class plus its dorsal counterpart is shown. Class A axons run forward in both cords whereas the class B axons run backwards. The dendritic branches of the ventral and dorsal class A neurones run in opposite directions as do those from class B neurones. Ventral class D neurons receive their synaptic input on the dorsal side and give neuromuscular synapses on the ventral side. The dorsal type D neurones have half the periodicity of their ventral counterparts and receive synapses on the ventral side and give neuromuscular synapses on the dorsal side. Class AS neurones only synapse on the dorsal side and have no ventral counterpart whereas the class C neurones only synapse on the ventral side. The synapses from class C motor neurones onto body muscles are less dense than for the other classes.
Motor neurones of the class AS only innervate the dorsal side. They receive the same synaptic inputs as type A motor neurones (being really a sub-class of A) and form gap junctions with them but they also receive additional chemical synapses from β-interneurones. Their axonal processes run anteriorly in the dorsal cord but their dendritic regions are very short and can run both anteriorly and posteriorly in the ventral cord. Often several short dendritic processes are seen emanating from the cell body.
Class D motor neurones receive their synaptic input from other motor neurones rather than from interneurones in the cord. The dendritic arms of these neurones are on the opposite side to the axons. Thus a ventral type D motor neurone forms n.m.js in its particular region on the ventral side while its dendritic process receives synapses in the same region but on the dorsal side, and is joined to the cell body in the ventral cord via a commissure. The dorsal type D neurones span twice the length of the ventral type Ds, have their dendritic fields on the ventral side and send out a commissure to the dorsal side which becomes an axon innervating the same region on that side. The cell bodies for both dorsal and ventral type Ds are situated at the posterior end of the process running in the ventral cord. The dendritic arms of class D neurons run above the three motor neurones on the ventral side (four on dorsal side) and usually send out short dendritic spines down into the region of the motor end-plate when one of the motor neurones forms a n.m.j. (figures 12, 13 and 14, plates 4 and 5). Sometimes one or other motor neurone will synapse directly onto a class D dendrite with no n.m.j. seemingly being involved (figure 16, plate 6). All classes of motor neurone extensively synapse onto the class D dendrite with the exception of the class D axon. This runs next to the dendritic arm but rarely seems to make direct synaptic contact with it (figure 15, plate 5). The anterior and posterior extremities of both the dendritic and axonal arms usually terminate in gap junctions to the corresponding dendritic or axonal arms of the adjacent type D neurone (figure 17, plate 6). Thus the extension of both the axonal and dendritic processes is restricted to the region in which they are active. This is unlike the other classes of motor neurone which although only forming n.m.js in well defined regions,. often have axons or dendrites which span a much greater distance. There are three type C motor neurones in the anterior ventral part of the cord; they are not present in the region of the r.v.g. which has members of all the other classes. Processes from all three end in the region of the r.v.g. or ventral ganglion at the anterior end, and go beyond the range of the reconstructions at the posterior end. They run together as a group (figure 14, plate 5) above the other motor neurones and make occasional synapses to muscles and the dendritic processes of the dorsal type D motor neurones. They differ from the other classes of motor neurone in that they form fewer n.m.js and do not have well defined non-overlapping regions where n.m.js are situated. They form gap junctions between themselves but nothing has been seen to synapse onto them in the anterior half of the animal. They also form extensive synapses onto the vulva1 muscle cells.
The synaptic connections in the ventral cord can be summarized in the form of a connectivity graph as shown in figure 21. This is a complete description of the synapses and gap junctions associated with the various classes of motor neurone in the anterior part of the ventral cord. Each motor neurone within a class conforms to this pattern. In the diagram gap junctions are shown between members of the same class; it should be emphasized that this is a strong feature, gap junctions are never seen between different classes of motor neurone.
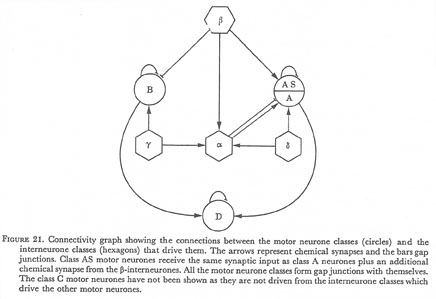 Figure 21. Connectivity graph showing the connections between the motor neurone classes (circles) and the interneurone classes (hexagons) that drive them. The arrows represent chemical synapses and the bars gap junctions. Class AS motor neurones receive the same synaptic input as class A neurones plus an additional chemical synapse from the β-interneurones. All the motor neurone classes form gap junctions with themselves. The class C motor neurones have not been shown as they are not driven from the interneurone classes which drive the other motor neurones.
Figure 21. Connectivity graph showing the connections between the motor neurone classes (circles) and the interneurone classes (hexagons) that drive them. The arrows represent chemical synapses and the bars gap junctions. Class AS motor neurones receive the same synaptic input as class A neurones plus an additional chemical synapse from the β-interneurones. All the motor neurone classes form gap junctions with themselves. The class C motor neurones have not been shown as they are not driven from the interneurone classes which drive the other motor neurones.
The type A motor neurones receive their gap junctions from alpha-interneurones while they are running along side them, but the type B motor neurones receive their gap junctions in the region of their cell bodies. This is because all the cell bodies of the cord are situated at the top between the hypodermal cell and the fibre bundle (figure 14, plate 5). In the vicinity of the cell body the processes from the motor neurones leave their normal positions in the fibre group and run up to the top to join the cell body, thus it is only in this region that neurones are situated next to interneurones and it is only here that type B cells receive their gap junctions, and type AS cells their chemical synapses from the B interneurones. Fibres from class A and B motor neurones run for a comparatively long distance when in the group under the alpha-interneurones with few connections at their distal ends. This is seen most strikingly for the first few motor neurones which run up anteriorly and stay in position in the 'corner' of the hypodermal cell after all the other fibres of the ventral cord have gone up into the ring. They eventually peter out well up in the head past the ring. The class D motor neurone axons, on the other hand, do not leave the neuromuscular region (except temporarily in the vicinity of the cell body on the ventral side) and end abruptly in a gap junction with the next class D in the series.
(g) Summary of neuromuscular circuitry
(a) Five classes of motor neurone are seen in the ventral cord; A, AS, B, D and C. These classes may be identified both on the basis of morphology of the member neurones and the synaptic inputs which they receive. Either criterion alone is sufficient uniquely to assign a class name to any given neurone.
(b) Class D motor neurones receive synapses from the motor neurones in the same region as they form n.m.js but on the opposite side, i.e. the dorsal type D neurones will receive synaptic inputs from ventral type A and B neurones and the ventral type D receive their inputs from dorsal type A, B and AS neurones in the same region.
(c) Class A, AS and B motor neurones receive their synaptic input from a set of interneurones in the ventral cord. Class A and AS are driven both by gap junctions and chemical synapses from the α-interneurones and chemically from the δ-interneurones. Class AS is additionally driven by chemical synapses from β-interneurones. Class B motor neurones are driven by gap junctions from β-interneurones and also weakly chemically by the γ-interneurones.
(d) Class C motor neurones do not receive any synaptic input in the cord but send processes down towards the tail. They are not as active as other motor neurones and are not present in the anterior region of the cord. They innervate the vulva1 muscles.
(e) Motor neurones of all classes form gap junctions with adjacent members of their own class.
(f) The motor neurone axons of a given region assemble themselves in a fixed position at the edge of the nerve cord when they are actively giving n.m.js together with the dendrite from the class D motor neurone of the opposite side. The order of processes on the ventral side is: class C axon, class D dendrite, class D axon, class A axon and class B axon (figures 12&13, 14&15 and 16&17, plates 4-6).
(g) The motor neurones of different classes have different polarities as defined by the direction in which the branch giving n.m.js leaves the cell body. On the ventral side, type A axons run anteriorly, type B posteriorly and type D anteriorly. On the dorsal side class A and AS axons run anteriorly, whereas class B and D axons run posteriorly (figure 20). The dendritic branches of the dorsal class A and B neurones run in the opposite direction to those on the ventral side, thus dorsal type A and B's are ' U ' shaped doubling back on themselves whereas the ventral type A's and B's are linear. Both ventral and dorsal type D neurones have processes on both sides and are also 'U' shaped.
(h) Motor neurones of a given class (except class C) have well defined fields where n.m.js occur. These fields do not overlap with the fields of adjacent neurones of the same class.
(h) Invariance
The three animals that were reconstructed in the anterior ventral cord had the same genotype (they were wild type N2 hermaphrodites, Brenner 1974), were grown up under identical conditions and were all fully mature adults containing fertilized developing eggs. Remarkably little difference was found in the structure of the ventral cords of these animals. The combination of cell position, morphology and synaptic activity is more than sufficient uniquely to identify a neurone in any given wild type animal. The variability that has been seen can be put into two categories.
(i) Synaptic variability
Tables 1, 2, 3, 4, and 5 show synapses which have been identified on neurones from each class, from the U series animal and from the S series. It can be seen that many of the connections are repeated, i.e. a neurone will generally synapse to another at more than one point. There is some variability as to the number of synapses a given motor neurone receives but generally the synapses obey the class rules that have been described. Some features such as the single gap junction seen between one A neurone (66) and the β-interneurone 4, and also the chemical synapse from 4 onto 79 do not obey these rules and seem to crop up variably as isolated instances. Similar observations have been made by Macagno, Lo Presti & Levinthal (1973) on the optic lamina of Daphnia.
The α-interneurones 1 and 3 form a bilaterally symmetrical pair in the ring as do the β-interneurones 2 and 4. However, often a tendency is seen to favour one member of a pair, thus 3 seems to make more gap junctions with type A's than 1, similarly 4 makes more gap junctions with type B's than 2. This is probably simply explained by the disposition of fibre in the cord (figure 12, plate 4), 3 and 4 lie nearer the regions where the motor neurones have their cell bodies and so presumably have more opportunity for making contacts with neurons of the appropriate class than their partners.
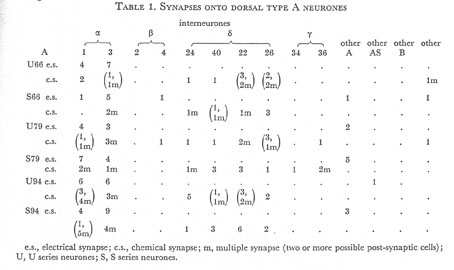 Table 1. Synapses onto dorsal type A neurones. e.s., electrical synapse; c.s., chemical synapse; m, multiple synapse (two or more possible post-synaptic cells) ; U, U series neurones; S, S series neurones.
Table 1. Synapses onto dorsal type A neurones. e.s., electrical synapse; c.s., chemical synapse; m, multiple synapse (two or more possible post-synaptic cells) ; U, U series neurones; S, S series neurones.
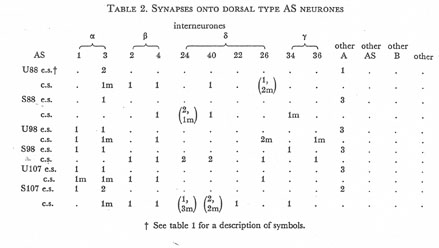 Table 2. Synapses onto dorsal type AS neurones. e.s., electrical synapse; c.s., chemical synapse; m, multiple synapse (two or more possible post-synaptic cells) ; U, U series neurones; S, S series neurones.
Table 2. Synapses onto dorsal type AS neurones. e.s., electrical synapse; c.s., chemical synapse; m, multiple synapse (two or more possible post-synaptic cells) ; U, U series neurones; S, S series neurones.
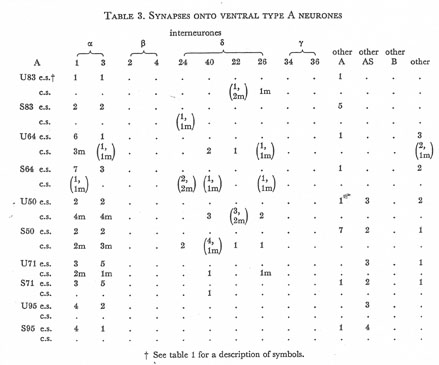 Table 3. Synapses onto ventral type A neurones. e.s., electrical synapse; c.s., chemical synapse; m, multiple synapse (two or more possible post-synaptic cells) ; U, U series neurones; S, S series neurones.
Table 3. Synapses onto ventral type A neurones. e.s., electrical synapse; c.s., chemical synapse; m, multiple synapse (two or more possible post-synaptic cells) ; U, U series neurones; S, S series neurones.
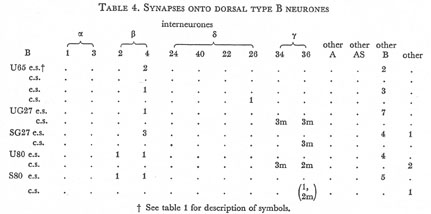 Table 4. Synapses onto dorsal type B neurones. e.s., electrical synapse; c.s., chemical synapse; m, multiple synapse (two or more possible post-synaptic cells) ; U, U series neurones; S, S series neurones.
Table 4. Synapses onto dorsal type B neurones. e.s., electrical synapse; c.s., chemical synapse; m, multiple synapse (two or more possible post-synaptic cells) ; U, U series neurones; S, S series neurones.
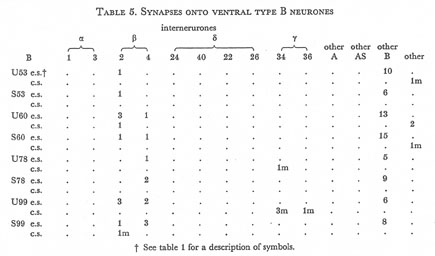 Table 5. Synapses onto ventral type B neurones. e.s., electrical synapse; c.s., chemical synapse; m, multiple synapse (two or more possible post-synaptic cells) ; U, U series neurones; S, S series neurones.
Table 5. Synapses onto ventral type B neurones. e.s., electrical synapse; c.s., chemical synapse; m, multiple synapse (two or more possible post-synaptic cells) ; U, U series neurones; S, S series neurones.
(ii) Morphological invariance
Figure 24 shows the relative positions of all neurones in the cord and r.v.g. in two of the series analysed. It can be seen that there is some variability in the relative positions of cells within the r.v.g. and rather less variability down the ventral cord, there being two switch-rounds of adjacent cells between the S and U series. The pattern of cells in the cord also has some common features, the gap after the r.v.g. followed by a pair is nearly always seen (J. Sulston, personal communication).
As nematode neurones have so few branches it is perhaps not too surprising that branching patterns are generally conserved, although some detailed variability is seen. Figures 22 and 23 show computer reconstructions of the AS motor neurone labelled 87 in the U and S series animals in the region of the cell body on the ventral side. This motor neurone is driven by synapses on a small dendritic arm which runs along for a short distance in the region under the α-interneurones where the other neurones of type A and B are grouped and receive their synapses. It also sends a commissure out to the dorsal side where it runs anteriorly forming n.m.js in its particular region. (This runs as a single unbranched process and is identical in both animals and so has not been shown.) As can be seen from the diagrams, in one case (the U series) the dendritic arm comes directly out of the cell body and the commissure comes out as a separate process, whereas in the other case it comes out as a branch of the commissural process. There is also an extra apparently non-functional branch seen in the U series which is not present in the S series. Thus it can be seen that the locations of processes which are synaptically active are invariant but the route which these processes take from the cell body is subject to some variation.
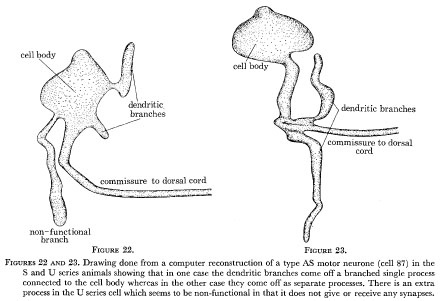 Figure 22 and 23. Drawing done from a computer reconstruction of a type AS motor neurone (cell 87) in the S and U series animals showing that in one case the dendritic branches come off a branched single process connected to the cell body whereas in the other case they come off as separate processes. There is an extra process in the U series cell which seems to be non-functional in that it does not give or receive any synapses.
Figure 22 and 23. Drawing done from a computer reconstruction of a type AS motor neurone (cell 87) in the S and U series animals showing that in one case the dendritic branches come off a branched single process connected to the cell body whereas in the other case they come off as separate processes. There is an extra process in the U series cell which seems to be non-functional in that it does not give or receive any synapses.
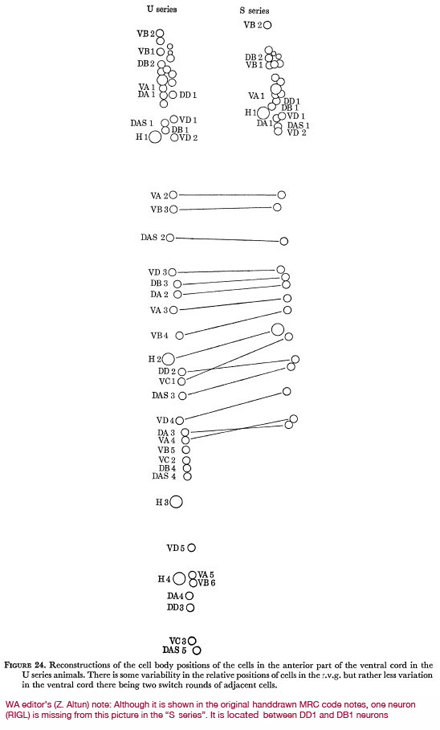 Figure 24. Reconstructions of the cell body positions of the cells in the anterior part of the ventral cord in the U series animals. There is some variability in the relative positions of cells in the r.v.g. but rather less variation in the ventral cord there being two switch rounds of adjacent cells.
Figure 24. Reconstructions of the cell body positions of the cells in the anterior part of the ventral cord in the U series animals. There is some variability in the relative positions of cells in the r.v.g. but rather less variation in the ventral cord there being two switch rounds of adjacent cells.
WA editor's (Z. Altun) note: Although it is shown in the original hand drawn MRC code notes, one neuron (RIGL) is missing from this picture in the "S series." It is located between DD1 and DB1 neurons.
Discussion
(a) How is it made?
(b) Guidance
(c) Polarity
(d) Lineage
(e) Competition
(f) Synaptic specificity
(g) How does it work?
The reconstructions which have been described have all been done on the anterior part of the cord. The longest series (the U series) covers just under one half of the probable total cord logic. J. Hodgkin has reconstructed some series that have been cut in the tail, covering the last segment and the beginning of the pre-anal ganglion, and it is possible to identify all five classes of motor neurone in the region on morphological criteria. It is not yet clear, however, whether the same set of interneurones are driving the class A, AS and B motor neurones in the head.
All the connections that have been described between the various classes of cell were allocated on morphological criteria alone. Mark, Marotte & Mart (1972) have suggested that morphologically normal looking synapses may not be functionally active in some instances. Neurones in C. elegans are so limited as to the extent and the diversity of their synaptic input that it seems unlikely that any particular group of synapses from one class of cell to another is 'silent'; however, this remains a possibility. It has been suggested that the vesicles in inhibitory synaptic terminals are more elongated than those from excitatory terminals (Uchinzono 1967). No consistent differences in vesicle morphology could be detected in the various classes of cells in the ventral cord so either none of the synapses described are inhibitory or more probably this criterion is not applicable to C. elegans.
Gap junctions are presumably the sites of electrotonic coupling between cells (Furshpan & Potter 1968), but again their morphological appearance gives little indication as to the electrical characteristics of this coupling.
(a) How is it made?
The structure of the ventral and dorsal cords of C. elegans has been described in some detail. Although this information does not provide unequivocal evidence for any particular way in which the structures might assemble themselves certain features do suggest possible mechanisms that may be used.
(b) Guidance
Neurones always lie in close apposition to hypodermal cells and are enclosed in a 'bag' of basement membrane. Muscle cells are situated on the other side of these membranes with neuromuscular connections apparently being made through them. It seems likely that the system of hypodermal cells with their associated basement membranes could act as a system of 'conduits' which constrain and guide growing nerve processes to grow along well defined tracts. It has been demonstrated that growing neurones will preferentially grow along ordered arrays of collagen filaments (Weiss 1934). The striations which have been seen in basement membranes might similarly provide some directional cues to growing fibres as they are oriented parallel to the fibre bundle. It might also be the case that these 'tram lines' have a polarity so that growing fibres can only follow them in one particular direction.
Growing processes may also take directional cues from existing fibres and follow them along. The observation that fibre bundles maintain their spatial coherence over long distances and that processes from a given class are grouped together is suggestive that this could be happening. Guidance along pre-existing fibres has been suggested by several authors (Harrison 1910; Trujillo-Cenoz & Melamed 1973; Lo Presti, Mecagno & Levinthal 1973).
(c) Polarity
The type A motor neurones have the opposite polarity to the type B motor neurones. The arms giving n.m.js from type A's run forward on both the ventral and dorsal sides. The dendritic arms from the dorsal and ventral members of each class do not run in the same direction however; the dorsal type A and the ventral type B run forward whereas the dorsal type B and the ventral type A run backward (figure 20). One possible explanation of this behaviour is that their axons (the branch that gives n.m.js) have a preferred direction of growth in the cords, being forward in the case of the type A and backward for the type B. This preference could be as a result of the interaction of the growing axon and the basement membrane which it will be assumed has an inherent polarity. The dendritic arms of the motor neurones run in the centre of the cord away from the basement membrane whereas the axons take up a position adjacent to these membranes when they give n.m.js. If one considers the direction of the dendritic branches, the dorsal members of a class can be seen to be inverted relative to the ventral members of the same class. When the dorsal members send out their axons into the motor end plate region next to the basement membrane it could be that they find they are growing in the wrong direction relative to the polarity written in the basement membrane and they then try to turn right round (like a shoot from a seed that is planted upside-down) and in doing so end up between the muscles and hypodermal cells where they are guided round as a commissure to the dorsal side to complete the turn and then can run up in their appropriate directions. Thus the only difference that there may be between dorsal and ventral members of the class A and B is that the dorsal ones are born upside-down relative to the ventral. Van der Loos (1965) has observed a similar behaviour of the pyramidal cells in the rabbit cerebral cortex. Cells which are 'improperly' oriented, i.e. upside-down, send out axons which double back and run in the normal direction whereas the dendrites run in the same direction relative to the cell body which is in the opposite direction to those from normally oriented cells. Axons from Maunthner's neurones have also been shown to have a preferred direction of growth (Hibbard 1965).
(d) Lineage
Certain cells in the ventral cord are grouped together in pairs; a dorsal type A neurone is usually next to a dorsal type B, a ventral type A is usually next to a ventral type B and a dorsal type AS is usually next to a ventral type D (figure 24). It seems likely that each of these pairs might be the daughter of a single precursor cell, thus the differentiation of the neurones into their respective classes would be occurring during or after this last division. This has shown to be the case for the ventral A and B pair and the D and AS pair (Sulston 1976). Class A and B neurones have intrinsic polarities which are opposite. The orientations of the two daughter cells (extrinsic polarity) must be in opposite directions so that if it is assumed that the divisions are in the transverse plane then there are two possibilities for the daughter cells: that their intrinsic and extrinsic polarities are aligned, or that they are opposed. In the first case both the axons find their direction of growth in accord with the polarity of the cord and innervate the ventral side, whereas in the second case this will not be so and they will turn and go into the dorsal cord where they will run in their preferred directions innervating the dorsal side.
(e) Competition
The different classes of motor neurones form repeating regions of neuromuscular contacts with fairly sharp points of demarcation where one member of a class ceases to give synapses and its neighbour takes over. These transition regions are not so precisely aligned between the classes (figures 18 and 19) and so it seems unlikely that there are any sort of precisely defined segmental boundaries constraining all neurones of a segment to form their n.m.js in that region. If that were so one might expect that the registration between the classes was as good if not better than that between adjacent members of the same class.
Competition between adjacent neurones has been postulated as a possible mechanism for the establishment of fields of innervation (Cajal 1929; Stirling 1970) and it seems likely that motor neurones within a given class compete with each other for sites in the n.m.j. region. The class D motor neurones and dendrites might be a more extreme example, where not only is the formation of neuromuscular connection inhibited by the presence of a neighbouring type D's but also the growth of the axon. The other classes of motor neurone send their axons well beyond the region where they form n.m.js but these branches seem to be completely non-functional being devoid of any synapses. The recognition of a neighbouring neurone of the same class and consequent partitioning off of territories may still be mediated gap junctions as it seems to be an almost universal rule in C. elegans that neurones of a given class form gap junctions with other members of its class. The formation of transient gap junctions between the growing fibres of the ommatidium and the neuroblasts of the optic lamina has been shown by Lo Presti et al. (1974). Cell contacts that are made via these gap junctions may serve to signal the ommatidial fibres to stop growing and even, as they suggest, to determine the fate of the target neuroblast cells.
(f) Synaptic specificity
It has been shown that the various classes of motor neurones receive their synaptic inputs from specific classes of interneurones, the rules being summarized by the connectivity graph of figure 21. It seems highly likely that some sort of chemical specificity exists and is giving rise to these rules. The dispositions of the cell bodies and dendrites of ventral class A and dorsal class B are identical as far as can be seen yet they receive different synaptic inputs. While it may be possible to postulate that a timing effect is producing these differences it seems far more likely that some sort of cell-cell recognition is taking place. The specificity seems only to exist between classes; neurones are fairly indifferent as to which members of a particular class they form connections. This seems to be determined by the mechanical dispositions of the fibres, connections being formed when two members of the appropriate classes come together. The specificities giving rise to the class connectivity rules are not absolute. Two instances have been cited where the rules break down. These synapses look fairly normal and are indistinguishable from synapses which obey the class rules. Thus it looks as if, once the formation of a synapse is initiated, a morphologically normal synapse is produced even if it is to be the 'wrong' class of cell. It seems therefore, as if the initiation is a probabilistic process, the probabilities varying according to the classes of cells involved.
(g) How does it work?
The small size of C. elegans presents severe technical problems for the investigation of the physiological and electrophysiological properties of the muscle cells; however Ascaris muscles have been fairly extensively investigated, notably by del Castillo and his co-workers. This work has been reviewed by De Bell (1965). Little is known, at the moment, of the physiology of C. elegans muscle but the close homologies which exist between both the structure of the muscles of the two animals and the nervous system innervating them leads one to suspect that the two may also be similar in their physiological properties.
Isolated preparations of Ascaris body muscles exhibit endogenous myogenic spike activity. This activity can be modulated by the application of acetylcholine which increases the spike frequency and y-amino-butyric acid (GABA) which decreases it. It is likely that these neurotransmitters are used by the various classes of motor neurone; the acetylcholine ones being excitatory and the GABA containing ones inhibitory. The spike activity in adjacent cells is strongly correlated with little or no latency suggesting that the muscle cells are coupled together electrically. This coupling is probably mediated by gap junctions such as those that are seen between muscle cells in C. elegans. Crofton (1966) has suggested on the basis of these observations that the waves of muscle contraction that travel down the body when the animal is moving forwards or backwards, are propagated myogenically, the muscles acting as their own proprioreceptors and the nervous system serving only to modulate this activity.
The synaptic circuitry associated with the various classes of motor neurone innervating the body muscles gives a certain amount of support for these ideas. None of the neurones described in the ventral cord have any structures that would suggest that they may be mechanosensory. If the nervous system were being used for wave propagation one might suppose that such cells would be necessary in order to provide feedback to the neural circuit driving the muscles of information on posture. Motor neurones of class A, B and AS are driven via their respective sets of interneurones from the central nervous system (c.n.s.) i.e. the nerve ring. Each member of a given class receives the same synaptic input regardless of its anterior/posterior position in the cord. It seems unlikely that these are used for the propagation of waves but rather that they are used for the global on/off control of wave generation and propagation by the c.n.s. Classes A and B are the only neurones present in both the dorsal and ventral sides and so one is probably excitatory and the other inhibitory. AS motor neurones are only present on the dorsal side and could be used to set up a certain posture, such as the deep ventral bend that usually accompanies the transition from backward to forward motion (R. Freedman, unpublished observations).
Class D motor neurones are not driven by the c.n.s. but rather from the other classes of motor neurone. They receive their synaptic input on the opposite side from the regions where they give n.m.js suggesting that they may form a system of reciprocal inhibition to ensure that the front and the back work antagonistically. It is probable that they are only used when the muscle system is first switched on and act to set the initial phases of the muscle oscillators.
There are fewer class C motor neurones than other types and in the hermaphrodite they predominantly innervate the vulva. They are more numerous in the male (Sulston 1976) and probably receive their synaptic input from the ganglia in the tail which are much larger and more complex than in the hermaphrodite. It is possible that they are involved in male copulatory behaviour and hermaphrodite egg laying.
The animals move forward by propagating sinusoidal waves backward along the body and, conversely, move backward by propagating forward directed waves. The end of the body, where the waves are progressing from, seems more active and vigorous than the other end which often seems to be passively following, as though there is a gradient of muscle activity which may be produced by a similar gradient of motor neurone activity. If the muscle cells have endogenous myogenic activity and are stretched activated, as are Ascaris muscles, then the muscle cells along the body will behave as a set of coupled oscillators. If there is a gradient of synaptic input along the body, the muscles with the highest excitation will oscillate with the highest amplitude and frequency and entrain all the more distal muscle oscillators causing waves to propagate away from the point of highest excitation. Thus, for forward movement there might be an anterior/posterior gradient of excitation and for backward movement this gradient would be reversed.
Acknowledgments
Thanks are given to Marilyn Anness for help with printing micrographs and Lois Edgar for drawing figure 4.
References
Albertson, D. G. & Thomson, J. N. 1976 The pharynx of Caenorhabditis elegans. Phil. Trans. R. Soc. Lond. B 275, 299-325.
Bird, A. F. 1971 The structure of nematodes. New York: Academic Press.
Brenner, S. 1974 The genetics of C. elegans, Genetics 77, 71-94.
Ramon y Cajal, S. 1929 Etude sur la neurogenese de quelques vertebres. In Studies on vertebrate neurogenesis. Springfield, Ill : Thomas (1960).
Crofton, H. D. 1966 Nematodes. London: Hutchinson University Library.
Croll, N. A. 1970 The behaviour of nematodes. London: Edward Arnold.
De Bell, J. 1965 A long look at neuromuscular junctions in nematodes. Quart. Rev. Biol. 40, 233-251.
De Bell, J., del Castillo, J. S. & Sanchez, U. 1963 Electrophysiology of the somatic muscles of Ascaris lumbricoides, J . Cell Comp. Physiol. 62,159-165.
Dowling, J. E. & Boycott, B. B. 1966 Organization of the primate retina; electron microscopy. Proc. R. Soc. Lond. B 166, 80-111.
Furshpan, E. J. & Potter, D. D. 1968 Low resistance junctions between cells in embryos and tissue culture. Current topics in developmental biology 3. New York : Academic Press.
Harrison, R. G. 1910 The outgrowth of nerve fibres. J . exp. Zool. 9, 787-794.
Hibbard, E. 1965 Orientation and directed growth of Mauthner's cell axons from duplicated vestibular roots. Exp. Neurol. 13, 289-301.
Hodgkgin, J. 1974 Ph.D. Thesis, University of Cambridge.
Levinthal, C. & Ware, R. W. 1972 Three dimensional reconstructions from serial sections. Nature, Lond. 236, 207-210.
Lo Presti, V., Mecagno, E. R. & Levinthal, C. 1973 Structure and development of neural connections in isogenic organisms (1) Cellular interactions in the development of the optic lamina of Daphina. Proc. nntn. Acad. Sci. U.S.A. 70, 56-71.
Mark, R. F., Marotte, C. R. & Mart, P. E. 1972 The mechanism of selective reinnervation of fish eye muscles. IV. Identification of repressed synapses. Brain Res. 46, 149-157.
Mecagno, E. R., Lo Presti, V. & Levinthal, C. 1973 Structure and development of neural connections in isogenic organism (11). Proc. natn. Acad. Sci. U.S.A. 70, 433-437.
Revel, J. P. & Karnovsky, M. J. 1967 Hexagonal array of subunits in intercellular junctions of the mouse heart and liver. J . Cell. Biol. 33, C7-Cl2.
Rosenbluth, J. 1965 Ultrastructural organisation of obliquely striated muscle fibres in Ascaris lumbricoides. J . Cell Biol. 25, 495-515.
Schneider 1860 uber die Muskeln und Nerven der Nematoden. Arch. Anat. Physiol. LPZ. 1860, 224-242.
Stirling, V. R. 1970 Central adaptation in the salomander spinal cord. J . Physiol. 210, 184P-185P.
Sulston, J. E. 1976 Post embryonic development in the ventral cord of Caenorhabditis elegans. Phil. Trans R. Soc. Lond. B 275, 287-297.
Trujillo-Cenoz, 0. & Melamed, J. 1973 The development of the retina lamina complex in muscoid flies. J . Ultrastruct. Res. 42, 554-581.
Uchinzono, K. 1967 Characteristics of excitatory or inhibitory synapses in the central nervous system of the cat. Nature, Lond. 207, 642-643.
Van der Loos, H. 1965 The 'improperly' oriented pyramidal cells in the cerebral cortex and its possible bearings on problems of growth and orientation. Bull. Johns Hopkins Hosp. 117, 228-250.
Ward, S., Thomson, J. N., White, J. G. & Brenner, S. 1975 Electron microscopical reconstruction of the anterior sensory anatomy of the nematode C. elegans. J . comp. Neur. 160, 313-338.
Ware, R. W., Clark, C., Crossland, K. & Russel, R. C. 1975 The nerve ring of the nematode Caenorhabditis elegans J. comp. Neur. 162, 71-110.
Weiss, P. 1934 In vitro experiments on the factors determining the course of the outgrowing nerve fibre. J . exp. 2001. 68, 393-448. |